Ubigene's AAV Helps Reveal New Perspectives in Non-Alcoholic Fatty Liver Disease Treatment

Background
Non-alcoholic fatty liver disease (NAFLD) is a major subtype of liver disease worldwide, closely associated with insulin resistance and obesity. NAFLD is characterized by excessive accumulation of lipids in the liver and hepatic steatosis. Fenofibrate, a widely used clinical peroxisome proliferator-activated receptor α agonist, can effectively improve the development of NAFLD, but its mechanism of action is not fully understood. So the authors found that fenofibrate can modulate the composition of gut microbiota in a high-fat diet (HFD)-induced NAFLD mouse model, and the changes in gut microbiota composition depend on the TFEB-autophagy axis. Furthermore, we discovered that fenofibrate significantly improves hepatic steatosis and activates the autophagy regulator TFEB. It is evident that the improvement of NAFLD by fenofibrate depends on the TFEB-autophagy axis.
Abstract
The research team led by Lanqing Ma from The First Affiliated Hospital, Yunnan Institute of Digestive Disease, Yunnan Clinical Research Center for Digestive Diseases, Kunming Medical University (Kunming, 650032, PR China) published a research paper titled "Fenofibrate improves hepatic steatosis, insulin resistance, and shapes the gut microbiome via TFEB-autophagy in NAFLD mice" in European Journal of Pharmacology. This study used adeno-associated viruses constructed by Ubigene to knockdown the TFEB gene, providing an in-depth elucidation of the potential mechanism of the TFEB-autophagy pathway in fenofibrate treatment of NAFLD[1].
Results & Discussion
In this study, the authors used adeno-associated viruses constructed by Ubigene to overexpress or knockdown the TFEB gene to build gene intervention mouse models. They explored the mechanism of the TFEB-autophagy pathway in fenofibrate's improvement of NAFLD. As shown in Figure 1, when TFEB was specifically knocked down in the liver, the activating effect of fenofibrate on NAFLD mouse livers was inhibited, while TFEB overexpression increased autophagy activity in NAFLD mouse livers. Figure 2 shows that fenofibrate's improvement of hepatic steatosis, reduction of triglyceride levels, and insulin resistance in NAFLD mice depends on TFEB expression. Figure 3 results indicate that the host gene TFEB influences the composition of gut microbiota.
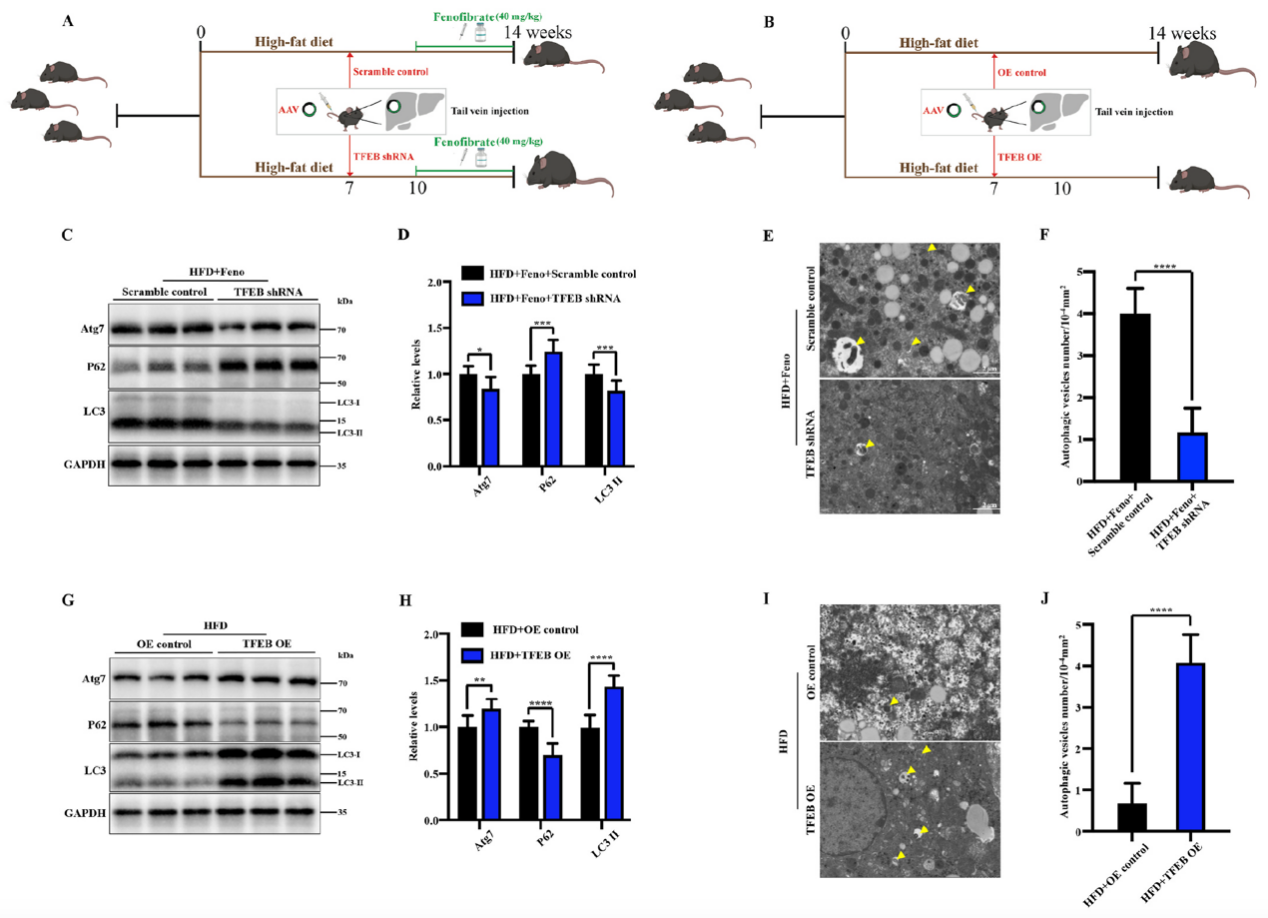
Figure 1 Fenofibrate induces autophagy in NAFLD mouse livers through TFEB
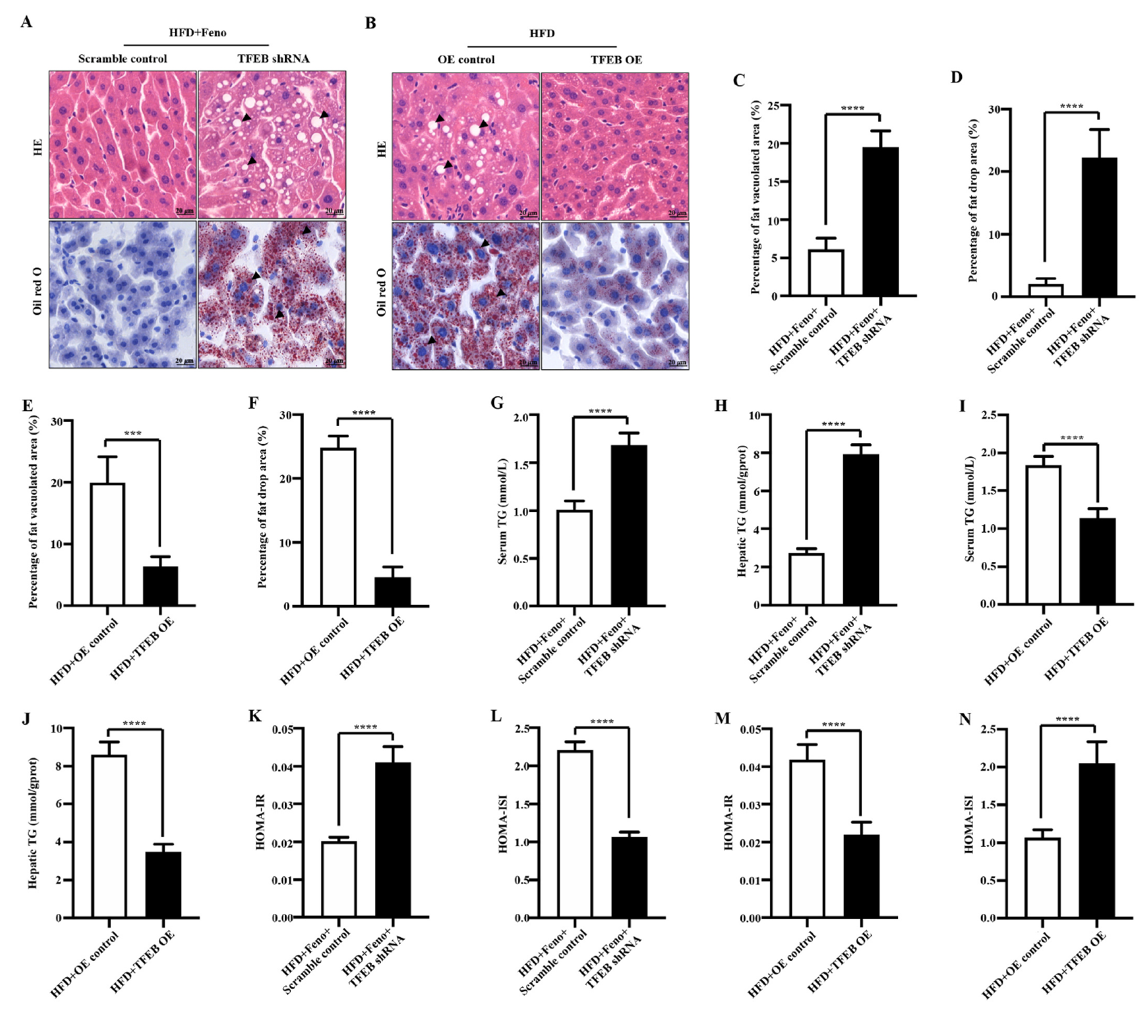
Figure 2 Fenofibrate improves hepatic steatosis, reduces triglyceride levels, and insulin resistance in NAFLD mice through TFEB
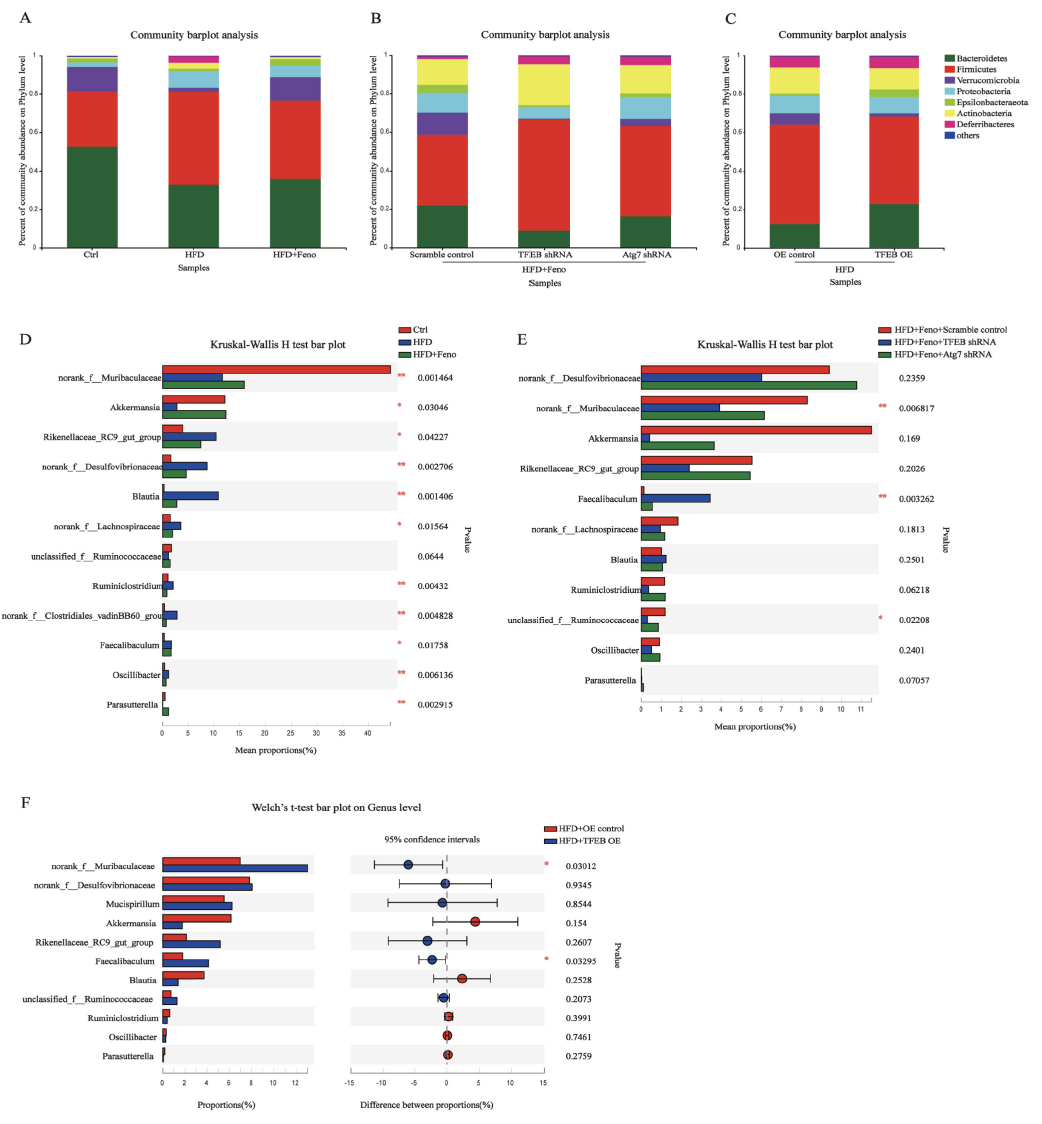
Figure 3 Fenofibrate influences microbial community composition through the TFEB-autophagy pathway
Conclusion
In summary, this study suggests that host genes may influence the gut microecology and emphasizes the role of TFEB-autophagy in improving NAFLD. This work expands our understanding of the mutual regulatory effects between the host and gut microbiota, providing new insights for alleviating obesity.
References
[1]Zhang D, Ma Y, Liu J, Wang D, Geng Z, Wen D, Chen H, Wang H, Li L, Zhu X, Wang X, Huang M, Zou C, Chen Y, Ma L. Fenofibrate improves hepatic steatosis, insulin resistance, and shapes the gut microbiome via TFEB-autophagy in NAFLD mice. Eur J Pharmacol. 2023 Dec 5;960:176159. doi: 10.1016/j.ejphar.2023.176159.