IF=10.3|Ubigene's Luc Cells Help Discover New Treatment for Liver Tumor Metastasis

Background
Toll-like receptor 9 (TLR9) agonists, as immunomodulators, can enhance the activity of checkpoint inhibitors (CPIs) in treating liver tumors. However, their effectiveness is somewhat limited by the unique biological properties of myeloid-derived suppressor cells (MDSCs) and challenges in tumor-specific therapeutic drug delivery. Researchers have shown that local delivery of C-type TLR9 agonists through a pressure-enabled drug delivery (PEDD) system can improve drug delivery to the tumor site and enhance the depletion of MDSCs. Overall, this drug delivery system can stimulate the immune system, with or without CPI combination.
Currently, CPIs are typically administered intravenously, but this method is time-consuming and costly. Therefore, researchers have begun to focus on the potentially advantageous subcutaneous (SQ) administration route.
Abstract
Recently, Chandra C Ghosh et al. published a research paper titled "Subcutaneous checkpoint inhibition is equivalent to systemic delivery when combined with nelitolimod delivered via pressure-enabled drug delivery for depletion of intrahepatic myeloid-derived suppressor cells and control of liver metastases" in Immuno Therapy of Cancer. The study used the MC38-Luc cells constructed by Ubigene to establish a mouse liver metastasis (LM) model. They found that when subcutaneous delivery of CPI was combined with PEDD system delivery of nelitolimod (a TLR9 agonist), the effect on controlling liver tumor development was comparable to systemic (Sys) administration routes. This suggests that the combination of subcutaneous CPI delivery and nelitolimod may be a reasonable option for treating liver tumors.
Results & Discussion
Researchers used the MC38-Luc cells constructed by Ubigene to establish a mouse liver metastasis (LM) model. MC38-Luc cells were injected into the spleen of C57BL/6 mice. After 7 days, fluorescently labeled nelitolimod (10 µg/mouse) was delivered via the PEDD system, combined with α-PD-1 administered systemically or subcutaneously. Tumor burden was monitored using an in vivo imaging system, and tissues were collected on day 3 or day 10 after PEDD to enrich CD45+ cells and analyze immune cell populations through targeted transcriptomics or flow cytometry.
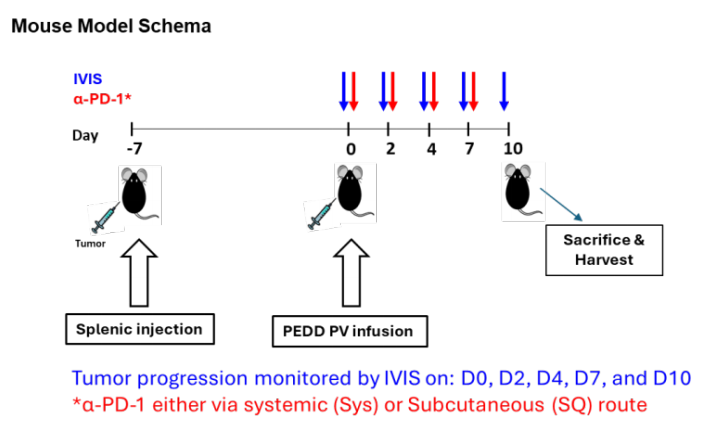
Figure 1: Experimental method
To assess whether the drug delivery method of CPI would affect its antitumor activity when combined with nelitolimod, researchers first delivered nelitolimod via PEDD in the LM mouse model, followed by Sys or SQ delivery of α-PD-1. Results showed that PEDD delivery of nelitolimod combined with either subcutaneous or systemic injection of α-PD-1 could effectively enhance the therapeutic effect on liver metastases.
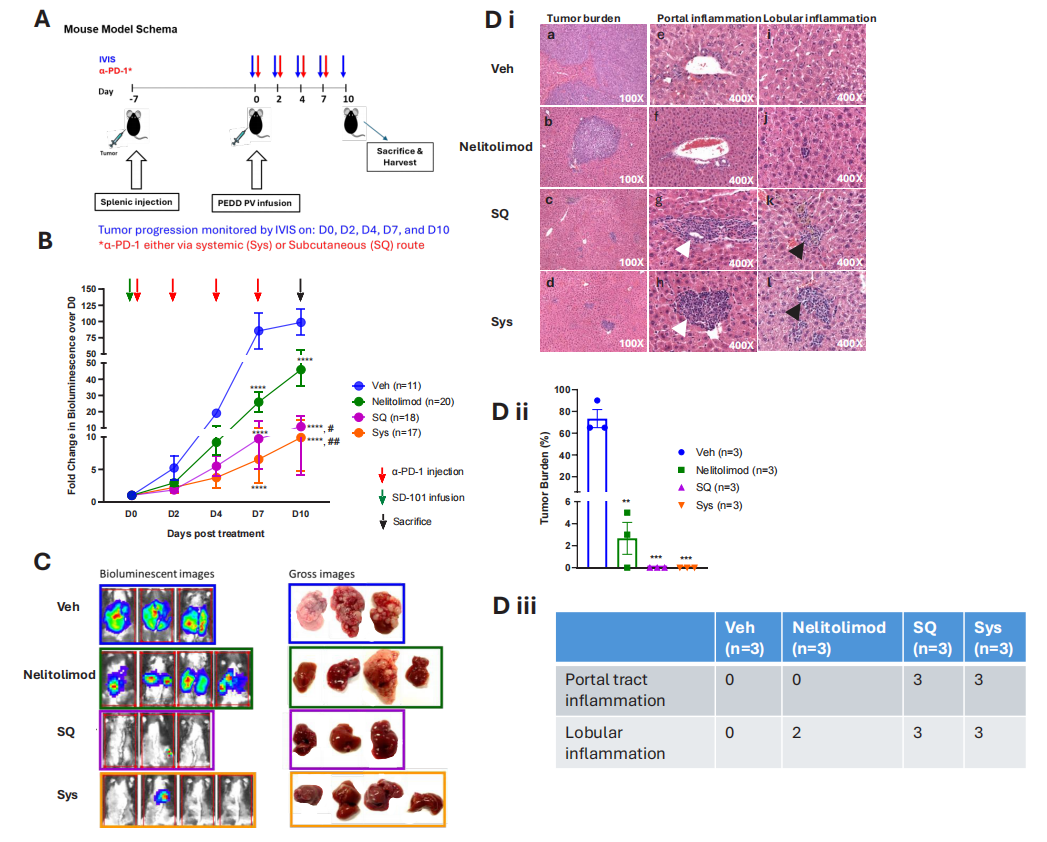
Figure 2: CPI enhances the antitumor activity of PEDD-delivered nelitolimod against liver metastases, regardless of the delivery method.
Researchers further explored the differential effects of various delivery methods on liver TME reprogramming. They harvested the livers of LM model mice on day 10 and isolated CD45+ cells from non-parenchymal liver cells. Through quantification of MDSCs and immune cell subgroup percentages and immunofluorescence analysis, results showed that when PEDD-delivered nelitolimod was combined with SQ or Sys α-PD-1, it could positively regulate immune cell populations in the liver. This includes reducing immunosuppressive myeloid-derived suppressor cells (MDSCs), especially the monocytic myeloid-derived suppressor cell (M-MDSC) subgroup, while increasing the number of antitumor M1-like macrophages, dendritic cells (CD11c+ DCs), B cells, and T cells. These changes in immune cell populations help enhance antitumor immune responses.
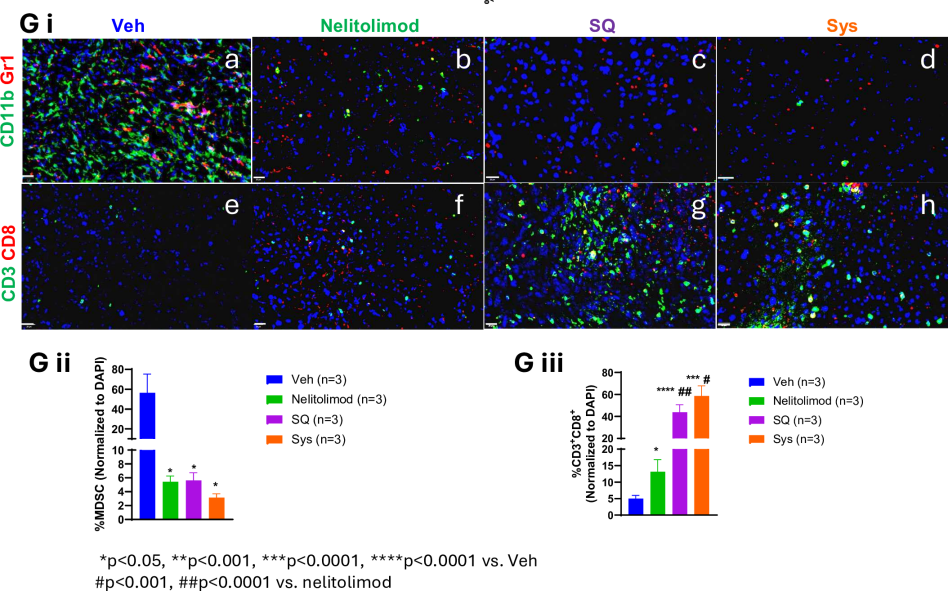
Figure 3: The regulatory effects of PEDD system-delivered nelitolimod on liver myeloid and lymphoid systems are maintained when combined with Sys or SQ delivery of CPI
To evaluate the peripheral pharmacodynamic effects of the PEDD system on nelitolimod, Luminex technology was used to analyze circulating cytokines in serum collected on day 3. Results showed that PEDD delivery of nelitolimod combined with SQ or Sys α-PD-1 increased levels of some immune response-related cytokines (such as IFN-γ and CXCL10) in peripheral blood, indicating activation of the immune system. Moreover, this treatment method did not adversely affect liver and kidney function in mice, suggesting good safety.
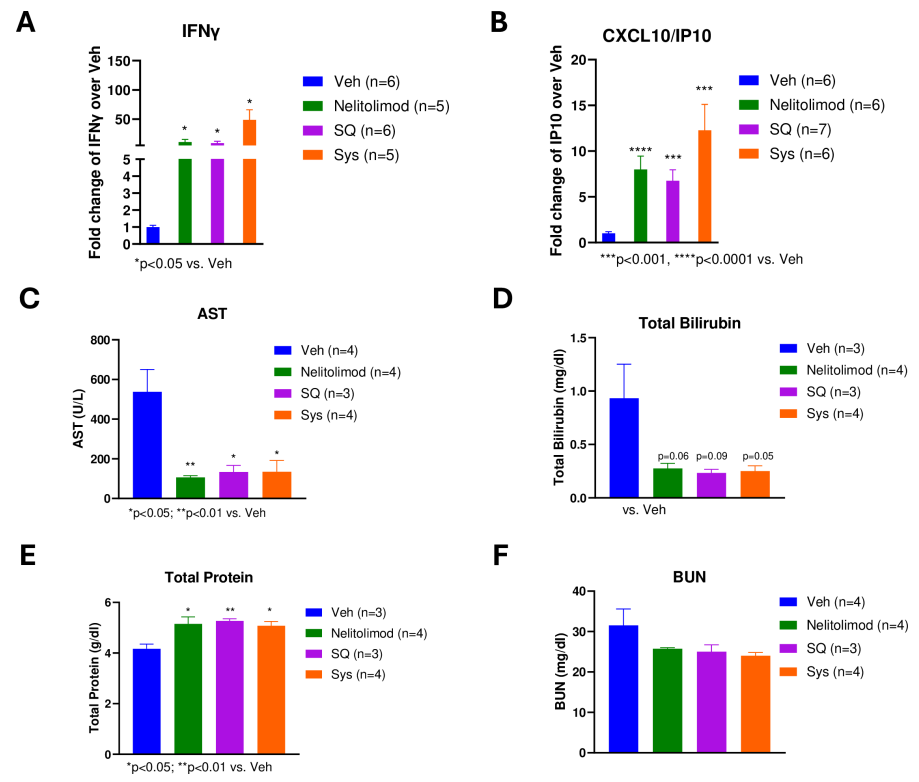
Figure 4: Peripheral effects of PEDD-delivered nelitolimod combined with Sys or SQ α-PD-1
From a gene expression perspective, the combination of PEDD-delivered nelitolimod with SQ or Sys α-PD-1 leads to changes in gene expression in the liver tumor microenvironment. These changes are favorable for enhancing antitumor immunity, including upregulation of genes related to interferon signaling, T cell function, and cellular immunity, and downregulation of genes associated with tumor cell migration, adhesion, differentiation, and metabolism unfavorable to immune responses.
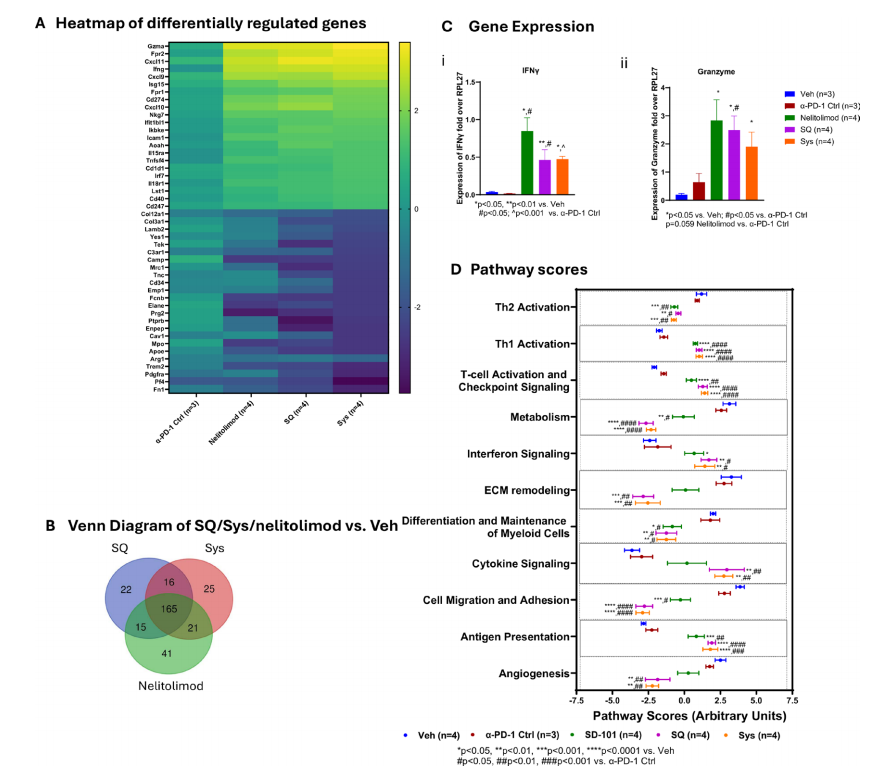
Transcriptome changes from PEDD-delivered nelitolimod combined with Sys or SQ α-PD-1 are consistent with enhanced antitumor immunity in the TME.
Conclusion
When subcutaneous delivery of CPI is combined with PEDD delivery of nelitolimod, the effect on controlling liver metastases is comparable to systemic delivery methods. This suggests that the combination of subcutaneous CPI delivery and nelitolimod may be a reasonable option for treating liver tumors.
References
Ghosh, Chandra C et al. “Subcutaneous checkpoint inhibition is equivalent to systemic delivery when combined with nelitolimod delivered via pressure-enabled drug delivery for depletion of intrahepatic myeloid-derived suppressor cells and control of liver metastases.” Journal for immunotherapy of cancer vol. 12,7 e008837. 22 Jul. 2024, doi:10.1136/jitc-2024-008837












