Expert Insights | Tutorial for THP-1 Cell Culture

In the previous session of Expert Insights, we introduced the tips for RAW264.7 cell culture, which received plenty positive feedback. We are so glad that we can help you all with our experience! Now, we present the 2nd session of Expert Insights for cell culture - THP-1. Get your notebooks and pens ready, the tutorial is starting now!
THP-1 is a monocytic leukemia cell line derived from the peripheral blood of patients with acute monocytic leukemia. Under the influence of certain differentiation inducers, THP-1 cells can be induced to differentiate into various macrophages, making it an ideal model for research in inflammation, immune responses, and other fields. In addition, THP-1 cells edited with CRISPR-Cas9 technology can be used for studies on macrophage mechanism, immune response pathways, and the development of treatments for inflammatory diseases, etc.
It is worth noting that THP-1 cells are suspension cells and prefer acidic environments. Their medium-changing and passaging procedures are quite different from adherent cells, so what are the differences? Keep reading to find out!
First, let's learn some basic information about THP-1.
Cell Name | THP-1 (Human monocyte leukemia cell line) |
Growth Characteristics | Suspension |
Cell Morphology | Monocytes, occasional small clusters during culture are normal |
Cell Culture Medium | 90% RPMI-1640 + 10% FBS |
Culture Environment | CO2:5%; Temperature: 37°C |
Medium Change Frequency | 2-3 times/week |
Passage Ratio | 2×105~4×105 cells/mL |
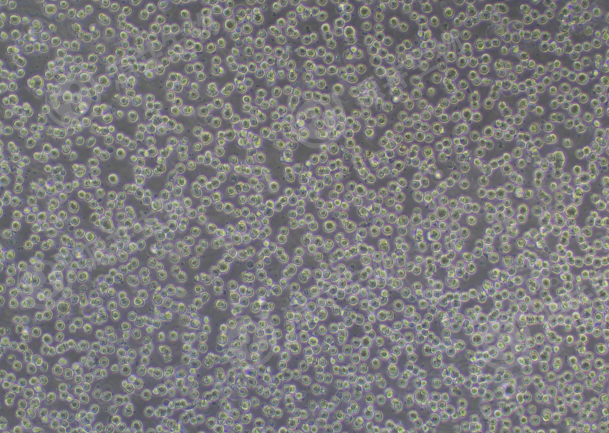
Figure 1. THP-1 Cell Image (Normal Growth)
Cell Thawing 1) Preparation: warm up the complete culture medium in
37°C water bath for 30 mins;
2) Inside the ultra-clean bench, pipet 7 ml of complete
medium into a 15 ml centrifuge tube;
3) Take out the cryopreserved vial from dry ice, hold
the cap with forceps, quickly thaw cells in a 37°C water bath by gently swirling the vial (Note: keep the cap out of
the water) for 1 minute to completely thaw the cells;
4) Transfer the thawed cells to the prepared centrifuge
tube with complete medium, close the lid, and centrifuge at 1100 rpm for 4 mins at room temp to collect the cells;
5) Inside the ultra-clean bench, carefully remove and
discard the supernatant. Resuspend cell pellet with 1ml of complete medium and then transfer to a T25 flask (or
6 cm plate) containing 4 ml of complete medium, label the flask/plate with cell name, date and passage no.,
incubate the flask in a 37℃, 5%CO2 incubator.
6) Observe the cell status and adhesion the next
day.
Medium ReplacementHalf medium replacement: Use when cells in good condition, with few cell debris and no yellowing of the culture medium
1) Allow the culture flask to stand upright for 10-15 min for the cells to settle naturally.
2) Carefully aspirate and discard half of the culture medium (if the cell density is low, transfer the aspirated half of the culture medium to a centrifuge tube and centrifuge at 1100 rpm for 4 minutes, then resuspend in fresh culture medium before returning to the original flask).
3) Refill the original flask with half of the fresh complete culture medium.
Centrifugation for medium replacement: Use when cells in good condition, with many cell debris and the culture medium turned yellow
1) Transfer all cell suspension to a centrifuge tube.
2) Centrifuge
at 1100 rpm for 4 minutes.
3) After
centrifugation, discard the supernatant, add 1 ml PBS for resuspension, transfer to a 1.5 ml EP tube, centrifuge
again (the purpose of this step is to remove cell debris; if
there are many cell debris, repeat the PBS washing 2 times, if
not, this step can be omitted).
4) Add an
appropriate amount of complete culture medium to the culture flask.
5) After
centrifugation, resuspend the cells in complete culture medium, seed the cells back into the culture flask, and
place in a 37°C, 5% CO2 incubator for culture.
Tips:
THP-1 cells thrive in
an acidic environment, they grow better in slightly acidic conditions. Therefore, when the
culture medium turns slightly yellow (orange-red), it is suitable for cell growth. At this point, a medium adding or
a half medium replacement is appropriate, avoid frequent centrifugation
for medium change.
Cell Passaging
1) Transfer all
cell suspension to a centrifuge tube, centrifuge at 1100 rpm for 4 minutes
2) After centrifugation, discard the supernatant, gently mix the cells with an appropriate amount
of complete culture medium, pipette 20 ul of cell suspension for cell counting
3) Adjust the cell density to 2×105~4×105 cells/mL based on the counting results,
seed the cells in different sizes of culture flasks according to the cell culture volume.
4) Place in a 37°C, 5% CO2 incubator for culture.
Direct splitting
method
1) Gently mix the cells in the
culture flask using pipette
2) Pipette
20 ul of cell suspension for cell counting
3) Based on the counting results, adjust the cell density to 2×105~4×105 cells/mL,
pipette the cell suspension from the culture flask, evenly distribute it into several new culture
flasks, and add an appropriate amount of fresh complete culture medium to each flask
4) Place in a 37°C, 5% CO2 incubator for culture.
Cell Cryopreservation
1) Transfer all cell suspension from the culture flask to a centrifuge tube
2) Centrifuge
at 1100 rpm for 4 minutes, discard the supernatant, add a suitable amount of pre-cooled freezing medium to resuspend
the cells, pipette 20 ul of cell suspension for cell counting, and adjust the cell density to at least
2×106~5×106 cells/mL.
3) Aliquot into cryopreservation tubes
at 1 mL per tube, place the cryopreservation tubes in a freezing
box, then transfer to a -80°C freezer
4) After
overnight, transfer the frozen cells to liquid nitrogen for long-term storage.
Tips:
1. THP-1 cells are sensitive to cryopreservation temperatures, it is recommended to
transfer to a -80°C freezer immediately after cryopreservation, for long-term storage, they should be
placed in liquid nitrogen.
2. It is recommended that the cell viability be greater than
90% during cryopreservation, with at least 2×106~5×106 cells/ml
per cryopreservation tube.
Troubleshooting common problems
1. Too many cell debris or
dead cells during cell culture or after antibiotic screening:
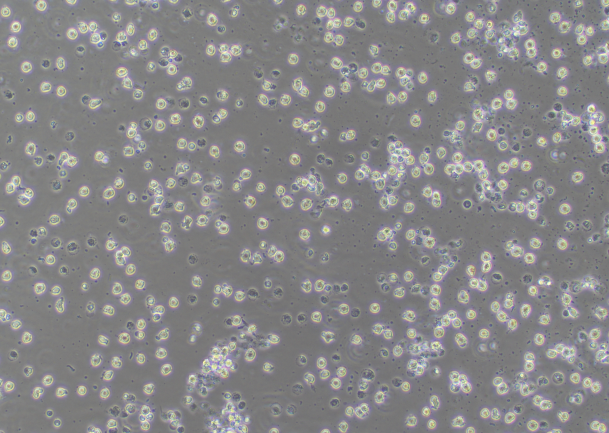
Figure 2. Many cell debris and dead cells observed
Circular, clear, plump,
non-shrinking cells are the living cells. If there are too many cell debris or dead cells,
after centrifugation, you can add an appropriate amount of PBS to resuspend the cells, transfer the cell suspension
to a 1.5 ml EP tube for low-speed centrifugation (800 rpm for 3 minutes), carefully remove PBS after
centrifugation. This step can be repeated for two times based on the amount of cells, then seed in a
suitable culture plate (preferably a small plate)
2. Cell clumps occur during cell culture:
A small amount of cell clustering is normal, but if there are too many clumps, consider
the following solutions:
1) THP-1 cells have certain requirements for the quality of serum, try switching to a better serum brand or increasing the serum concentration (not
exceeding 20%, excessive serum content can also be toxic to cells)
2) Control the cell density well, not too sparse or too dense
3 . Low viability after cryopreservation and thawing
(low survival rate):
1) Because THP-1
cells are suspension cells, they are sensitive to cryopreservation, so it is recommended to increase the cell
density during cryopreservation to improve the survival rate of thawed cells, it is recommended to have a cell
density of at least 2×106~5×106 cells/mL during cryopreservation.
2) If unsure about the number of cryopreservation cells during thawing,
you can resuspend the cells in a well of a 6-well plate, transfer them to a larger plate after the cell
condition has recovered.
Tips for Gene-Editing
Don’t let the sharing stop! Here are some tips for you based on our rich experience on gene-editing THP-1 successful cases:
1. How to improve single clone formation rate?
1) Cell viability needs
to be >90% when isolating clones
2) The cell counting result after dilution is best between 1×106-2×106
3) It is recommended to use premium fetal bovine serum
4) When isolating clones, it is recommended to wash with PBS twice
2. What to notice during cell transfection?
1) Cell viability > 90%, cells should be in good condition, visually they are round, transparent, and have intact cell membranes
2) For viral infection method, it is recommended to conduct a pre-experiment to find the optimal MOI and antibiotic screening concentration before formal experiments; ensuring that the titer of the infecting virus meets standards; add the transfection-enhanced agent Polybrene before infection; infection is best performed in a 24-well plate or 12-well plate
Another day full of harvest of knowledge! The quality of THP-1 cells is essential for successful experiments. Ubigene’s THP-1 cell line was preferbly selected through hundreds of gene-editing experiments. Ubigene can also provide gene-editing technology services on THP-1 and other 200+ cell lines. Contact us now to easily obtain high-quality THP-1 cell products and customized services!