Nat Commun. | Ubigene's gene-editing microbe reveals the membrane translocation mechanism of secretins

Bacterial type II secretion system (T2SS) widely exists in a variety of Proteobacteria, including non-pathogenic Escherichia coli (E. coli), Enterotoxigenic E. coli (ETEC), Enteropathogenic E. coli (EPEC), etc. T2SS substrate can promote bacteria to absorb nutrients from the environment or symbiosis with plants or animals in non-pathogenic bacteria. While in pathogenic bacteria, T2SS substrate can help adhere to the host, intoxicate host cells, and inhibit the immune function of the host, causing a variety of diseases. Since T2SS substrates have diverse functions and are closely related to virulence and diseases, it is necessary to understand their structure and mechanism of action for understanding bacteria functions and formulating antimicrobial strategies.
Xiaodong Shi et al. from Xuzhou Medical University, China published a research paper entitled “Membrane translocation process revealed by in situ structures of type II secretion system secretins” (IF=16.6) on Nature Communications. The study used in situ electron cryotomography (cryo-ET) in wild-type and GspS knockout Escherichia coli (constructed by Ubigene) and reported the in situ structures of two types of T2SS secretins, the Klebsiella-type GspDα and the Vibrio-type GspDβ and revealed the translocation mechanism of these two distinct secretins[1].
The results showed that under the condition of the overexpression of GspDα, GspDα exists in two different conformations on the inner membrane (Fig. 1b-c) and is in unstable state with significant mobility, which helps it be further transported to the outer membrane; Under the high concentration of D-methionine, peptidoglycan crosslinking decreases, and GspDα will be translocated to the outer membrane, forming evenly-distributed connections with the outer membrane, and GspDα adopts a more consistent conformation (Fig. 1a).

Figure 1. In situ structures of the GspDα secretin on the outer and inner membranes.
When overexpressing another secretin, GspDβ and a small protein GspS, particles are all found on outer membrane in the form of GspDβ-GspS complex (Fig. 2a-b); While overexpressing GspDβ only, most particles are located on the outer membrane (Fig. 2c) with a small amount of GspDβ particles are located on the inner membrane (Fig. 2d), which showed that without the expression of exogenous GspS, the endogenous GspS of E. coli is sufficient to locate GspDβ to the outer membrane, where GspS stays attached to GspDβ; When GspS is knocked out, GspDβ particles all appear on the inner membrane (Fig. 2f), and in its in situ structure, the density of GSPS could not be observed (Fig. 2g), which indicated that GspDβ can self-assemble into a multimer with a stable conformation on the inner membrane.
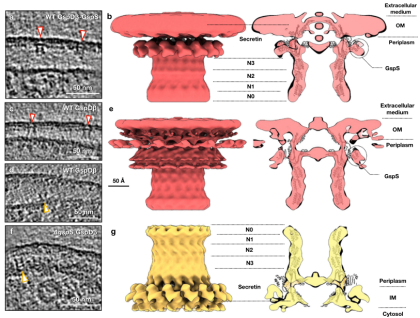
Figure 2. In situ structures of the GspDβ secretin on the outer and inner membranes.
This study captured the in situ structure of two secretins of E. coli T2SS in the process of membrane translocation and revealed the translocation mechanism of the two secretins, increased the understanding of the membrane interaction of T2SS secretins and their membrane translocation process, and provided a new perspective for the process of secretin biogenesis.
References
[1] Yu Z, Wu Y, Chen M, et al. Membrane translocation process revealed by in situ structures of type II secretion system secretins[J]. Nature Communications, 2023, 14(1): 4025.








