Research Highlight | Ubigene’s gene knockout cell line helps uncover the molecular mechanism of p53-BCL-2 interaction
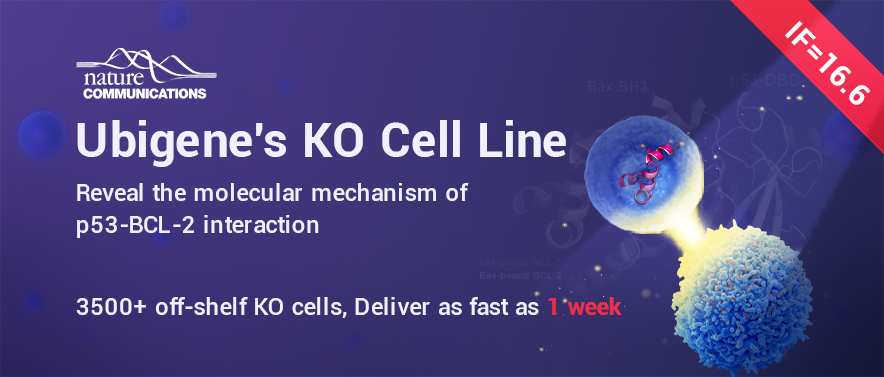
Tumor suppressor gene TP53 is the most frequently mutated gene in the process of tumorigenesis, and TP53 gene mutations are detected in more than 50% of human tumors. P53 protein is activated under cellular stress conditions such as DNA damage, and regulates a variety of cellular processes, including cell cycle arrest, inhibition of cell proliferation, and promotion of apoptosis. P53 and B-cell lymphoma-2 (BCL-2) protein family play a crucial role in mediating apoptosis. P53 can not only regulate the expression of apoptosis related genes as a transcription factor, but also play a transcription independent role in regulating mitochondrial apoptosis through direct interaction with BCL-2 family proteins. However, at this stage, little is known about the molecular mechanism of the interaction between p53 and BCL-2 family proteins.
Recently, Yongheng Chen’s research group from Xiangya Hospital, Central South University published an original research paper entitled "Structures of p53/BCL-2 complex suggest a mechanism for p53 to antagonize BCL-2 activity" on Nature Communications (IF: 16.6). In this study, the HCT116 cell line with TP53 gene knocked out constructed by Ubigene was used. By analyzing the crystal structure of the complex of p53 and the anti-apoptotic protein BCL-2, and combining biochemical and cellular experiments, the molecular mechanism of how p53 and BCL-2 proteins regulate apoptosis through interaction was deeply elucidated [1].
In this study, the authors first used X-ray diffraction to obtain the crystal structure of p53 and BCL-2 complex with a resolution of 2.5 Å. The complex structure revealed that the two flexible loops of p53 were directly bound to the BH3 hydrophobic binding pocket on the surface of BCL-2 protein (Figure 1). Asp186, Leu188 and Leu201 of p53 protein located at the interaction interface and Phe104, Arg107, Tyr108 and Arg146 of BCL-2 protein played key roles in protein-protein interaction. GST pull-down and MST experiments related to mutations also verified this conclusion.
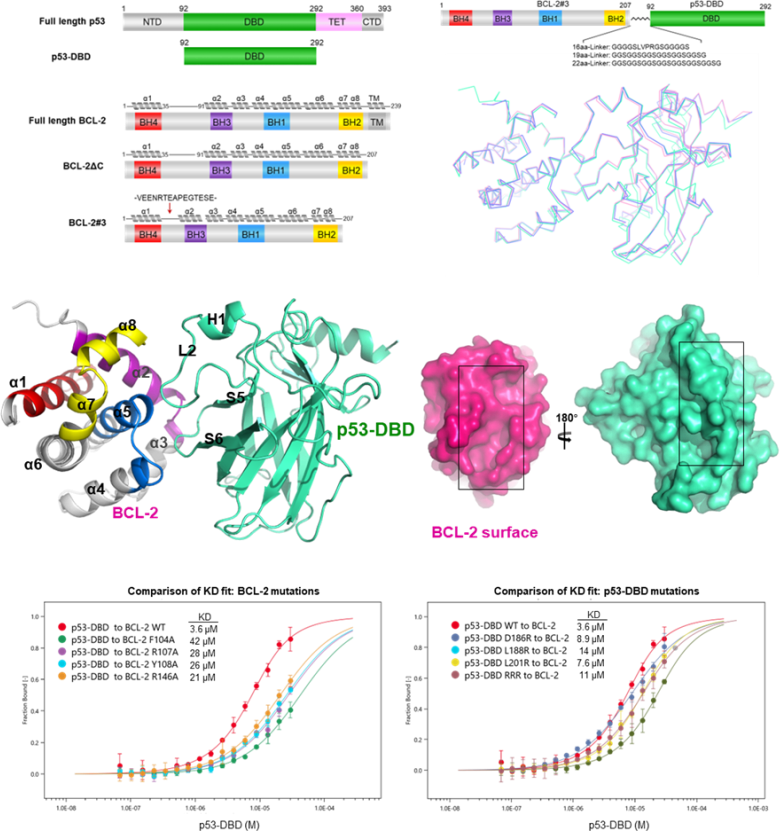
Figure 1. Structure of p53 and anti-apoptotic protein BCL-2 complex
Further structural analysis revealed that this BH3 hydrophobic binding pocket of p53 on the surface of BCL-2 is the main region of pro-apoptotic BCL-2 family members (such as the pro apoptotic protein Bax), and also the targeting position of clinical targeted drugs (such as Venetoclax). In order to further study how p53/BCL-2 interaction regulates apoptosis, the authors then verified that p53 can competitively bind BCL-2 with Bax and other pro-apoptotic proteins through fluorescence polarization experiments and microscale thermophoresis experiments, so that Bax and other pro-apoptotic proteins can be released to promote mitochondrial outer membrane permeabilization (MOMP) (Figure 2). In addition, the authors designed a p53 mutant (Ti-p53RRR) with defective interaction with BCL-2 on the basis of transcription defective p53 (Ti-p53). Flow cytometry experiments showed that p53-/-HCT116 cells transfected with Ti-p53RRR had significantly reduced apoptosis compared with Ti-p53 (Figure 3).
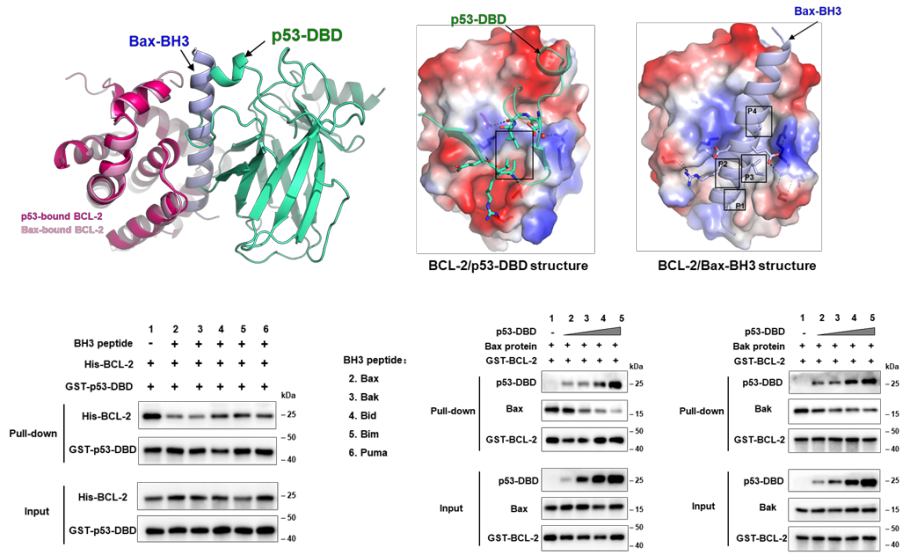
Figure 2. p53 competitively inhibits the interaction of BCL-2 with pro-apoptotic BCL-2 family proteins
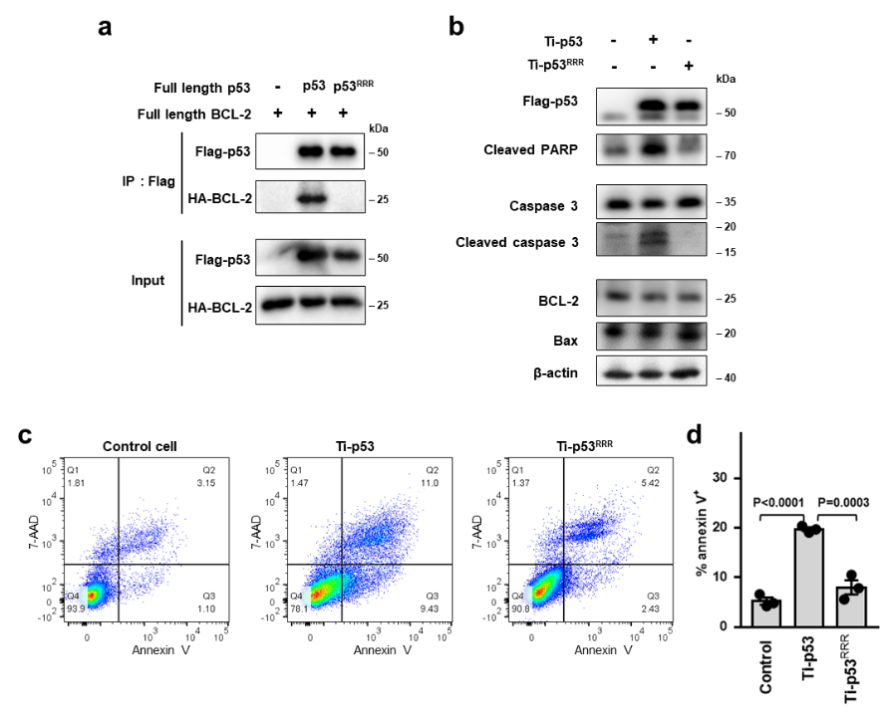
Figure 3. Mutations in p53 interacting with BCL-2 reduce apoptosis
In summary, this study revealed a new mechanism of p53 interacting with BCL-2 protein and promoting apoptosis, that is, p53 forms a complex with BCL-2 by directly occupying the BH3-binding pocket of BCL-2, and antagonizes BCL-2 activity by releasing pro-apoptotic BCL-2 family proteins located in the pocket, thereby promoting apoptosis. These structural and functional data provide a new idea for further understanding the complex regulatory mechanism of p53-mediated mitochondrial apoptosis, and also provide an important basis for developing anticancer therapeutic strategies that target protein-protein interactions to activate apoptosis.
Reference
[1] Wei H, Wang H, Wang G, Qu L, Jiang L, Dai S, Chen X, Zhang Y, Chen Z, Li Y, Guo M, Chen Y. Structures of p53/BCL-2 complex suggest a mechanism for p53 to antagonize BCL-2 activity. Nat Commun. 2023 Jul 18;14(1):4300. doi: 10.1038/s41467-023-40087-2.









