Published on: March 28, 2024
Views:
--
IF:17.1| Luciferase Mouse: Boosting Cancer Immunotherapy

Optical in vivo Imaging is an important tool in recent years for studying physiological and pathological processes in living organisms, including two main techniques: bioluminescence imaging and fluorescence imaging. Bioluminescence imaging uses the luciferase gene to label cells or DNA, while fluorescence imaging uses fluorescent reporter groups (such as GFP, RFP) or fluorescent dyes for labeling. Among them, fluorescence imaging requires an external light source to excite emission light, but many tissues and organs in vivo produce non-specific fluorescence after excitation, causing strong background noise and reducing detection accuracy. Bioluminescence, on the other hand, generates light through the reaction of the enzyme and substrate, with lower background noise, better sensitivity and specificity, and easier quantification. Next, let us take you through an application of bioluminescence imaging technology, luciferase imaging in mice, using the Ubigene’s Luciferase stable cell line (B16-F10-Luc cells) to construct a mouse tumor model, aiding in cancer immunotherapy.
| Bioluminescence Imaging | Fluorescence Imaging |
| Label | Luciferase gene | Fluorescent reporter groups (e.g. GFP, RFP) or fluorescent dyes |
| Light Emission Condition | Enzyme-substrate reaction | Requires excitation light |
| Characteristics | Good specificity, convenient quantification | High background noise, low sensitivity |
Table 1. Comparison of different optical in vivo imaging techniques
Cancer is one of the leading causes of death and a major obstacle to extending the human life expectancy globally. Currently, chemotherapy is a widely used cancer treatment method. Oxaliplatin (OXA) is a front-line anti-cancer drug that can effectively inhibit tumor growth and is clinically applicable for the treatment of various cancers. According to reports, OXA treatment can induce immunogenic cell death (ICD) of tumor cells, effectively activating the body's immune system, further enhancing its anti-tumor effect. However, the efficacy of OXA monotherapy in cancer treatment is not ideal, and the tumor immune-suppressive microenvironment (TIM) is an important factor limiting the effectiveness of ICD. Surviving tumor cells exposed to OXA may exhibit some in vivo immune escape reactions to resist the cytotoxic effects of immune effector cells.
Recently, researchers from the Institute of Biomedical Engineering, Chinese Academy of Medical Sciences, and Sun Yat-sen University published an original research article titled “Adaptive Design of Nanovesicles Overcoming Immunotherapeutic Limitations of Chemotherapeutic Drugs through Poliovirus Receptor Blockade” on ACS Nano (JCR Q1 IF:17.1). The authors developed a novel engineered drug-loaded cell membrane nanovesicle OXA@TIGIT MVs for cancer immunotherapy. The study used the Luciferase stable cell line constructed by Ubigene (mouse skin melanoma cells, B16-F10-Luc) for monitoring cancer onset and progression.
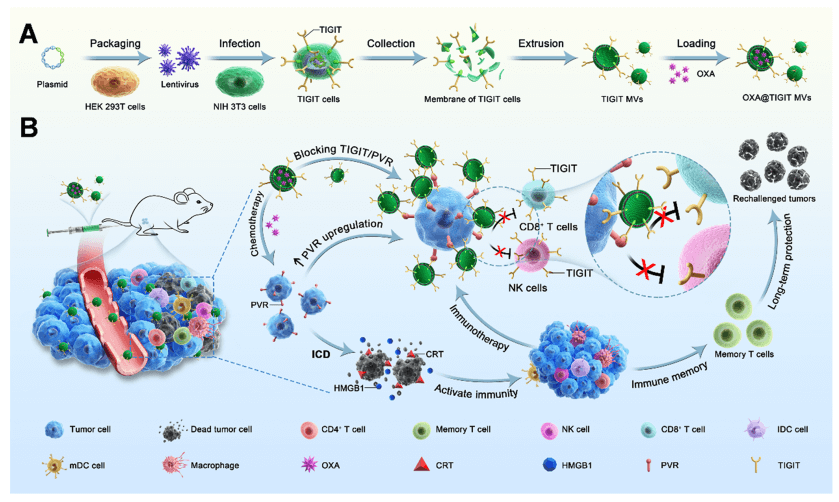
Figure 1. Schematic illustrating the preparation of OXA@TIGIT MVs and their anti-cancer effects.
In this study, the authors systematically investigated the specific effects of OXA on the TIGIT/PVR pathway, including the impact of different concentrations of OXA on PVR expression in different types of tumor cells, the relationship between tumor cell viability and PVR expression, and changes in TIGIT expression in immune cells. The authors found that the expression of PVR on tumor cells was further upregulated after OXA treatment, with a concentration-dependent effect. Based on this, through genetic engineering, stable cell lines with high TIGIT expression were prepared, and engineered cell membrane nanovesicles expressing TIGIT and loaded with OXA (OXA@TIGIT MVs) were further developed for effective synergistic therapy in cancer chemotherapy and immunotherapy.
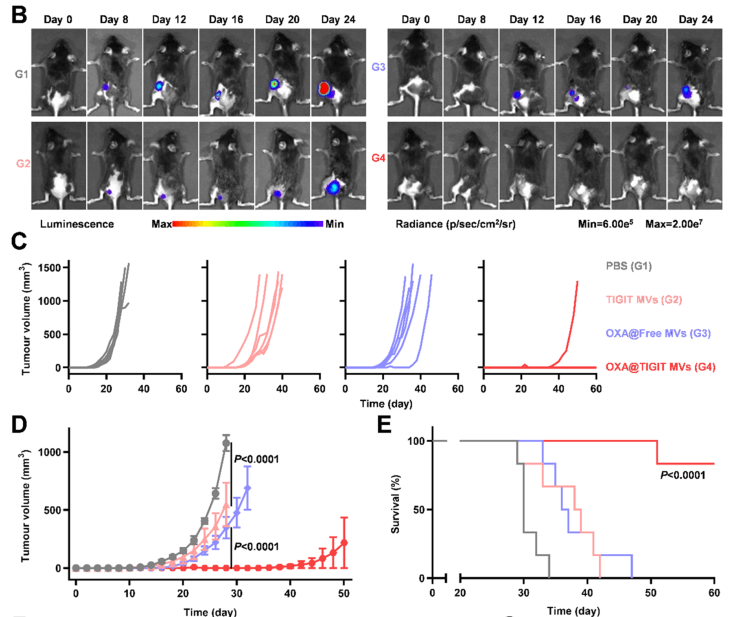
Figure 2. Evaluation of the suppressive effect of re-vaccination on tumor growth
OXA@TIGIT MVs exhibited excellent anti-cancer effects in multiple tumor models, effectively inhibiting tumor growth. Furthermore, the authors investigated immunological memory. The authors subcutaneously implanted tumors in one side of C57 mice, treated with different treatment formulations, surgically removed the original tumors, and about one month later, re-implanted tumor cells subcutaneously on the other side of the mice to assess the immunological memory induced by OXA@TIGIT MVs. In this process, the B16-F10-Luc cell line constructed by Ubigene was used to construct the tumor re-challenge model in mice, allowing for a clearer observation of the occurrence and development of re-implanted tumors. As shown in Figure 2B, the bioluminescent signal from B16-F10-Luc cells clearly showed the growth status of re-implanted tumors in each group of mice. The experimental results demonstrated that OXA@TIGIT MVs prevented tumors in the B16-F10 melanoma mouse model long-term, maintaining long-term immunity against cancer cells in the body, significantly inhibiting the growth of re-implanted tumors, and extending the survival of animals. Considering the team's series of related works and the successful delivery of liposomal drugs, the future of OXA@TIGIT MVs looks bright and hopeful, providing a new insight and direction for cancer treatment.
The combination of luciferase cell lines with imaging technology provides powerful tools for biomedical research, helping researchers better understand biological processes, disease mechanisms, and the efficacy of drug treatments. Ubigene’s Luciferase Stable Cell Lines have low passage numbers, high activity, and good cell condition, and can be directly used for in vivo cell injection, effectively assisting in transcription factor regulation mechanism studies, in vivo imaging, cell tracking, and other experiments. Ubigene not only provides in-stock luciferase cell line products but also offers customized luciferase cell line services. Consult us for more information!
References
[1]Yu, Yongkang, et al. "Adaptive Design of Nanovesicles Overcoming Immunotherapeutic Limitations of Chemotherapeutic Drugs through Poliovirus Receptor Blockade." ACS nano (2024).









