Having trouble with cell transfection? Did you pick the right
method?

Transfection is a very common operation in
cell experiments. Through transfection, foreign nucleic acids (DNA, RNA, etc.) can be introduced
into eukaryotic cells to study the function of genes, which is an essential step in the
construction of gene-editing cell lines. However, the problem of low transfection efficiency
always bothers many novices, and the transfection of a new cell line is usually challenging for
an experienced scientist.
What factors are involved in the efficiency of cell
transfection? Generally speaking, it can be summarized into three aspects:
1. Cells: Including the type, source and passage of the
cell line, which basically determines whether the cell line is easy to be
transfected.
2. Transfection vector: The size, quality and
quantity of the transfection vector also have impacts on the transfection efficiency. For
vectors of different sizes of the vectors, the experimental parameters are usage should be
optimized. In terms of quality, we should control the concentration, purity and conformation of
the plasmid, and check whether RNA is degraded.
3. Transfection methods and
reagents: no
one transfection method is applicable to all cell lines. For different cell lines, it is
important to select appropriate transfection methods and reagents.
In this article, we will summarize the
transfection methods and reagents, and share our experience with you.
First of
all, let's take a quick look at the methods of cell transfection. According to the principle of
transfection, it can be divided into three categories: physical
mediation (such as electroporation, microinjection, and
particle bombardment), chemical mediation (such as calcium
phosphate co-precipitation, liposome transfection, and cationic polymer mediation), and biological
mediation (mainly various viruses transduction). Among
them, the most commonly used
methods in laboratories are electroporation, liposome transfection and lentivirus
method.
Liposome transfection:
Liposomes
form a complex containing DNA molecules through the interaction between the positive charges on
the surface and the negative charges of nucleic acid phosphate groups. The complex is positively
charged on the surface and will be adsorbed by the negatively charged cell membrane, and then
complete the introduction of foreign DNA molecules through fusion or cell endocytosis, which is one of the
most convenient transfection methods in the laboratory at
present.
Following is
the detail process:
1) When the transfection reagent and nucleic
acid are incubated in vitro, a positively charged complex is formed.
2) The complex is added to the
cells and binds to the negatively charged cell surface
through electrostatic interaction.
3) The cells internalize the complex into
the membrane vesicles of the endosome through endocytosis.
4) Transfection reagent made the endosome
membrane unstable.
5) The complex escapes from the endosome and
releases nucleic acids in the cytoplasm (siRNA, miRNA and large RNA are usually active in the
cytoplasm).
6) DNA must be located in the nucleus to express and transcript.
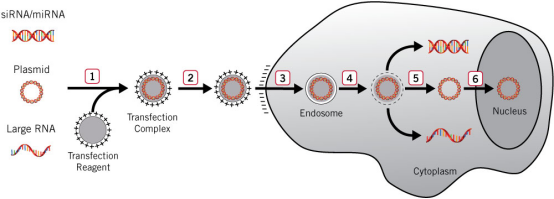
Figure 1. Liposome transfection
process [1]
Electroporation:
The
electroporation causes the change of cell membrane potential through high intensity electric field,
instantly improves the permeability of cell membrane, and makes the cell membrane produce reversible
pores to facilitate the entry of foreign nucleic acids (Figure 2). It is applicable
to almost all types of cells.
At present, the commonly used electroporation
systems on
the market include Neon of Thermo, Nucleofector series of Lonza, Bio-rad
Gene Pulser Xcell system, and Celetrix.
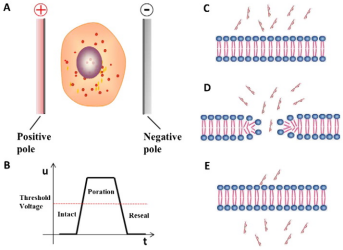
Figure
2. Principle of electroporation [2]
Lentivirus mediated transduction:
Lentivirus
can transduce non-dividing cells and dividing cells. Human immunodeficiency virus type 1 (HIV-1)
is the most studied lentivirus. The specific process is as follows:
1) The
target plasmid of genetic engineering transformation and the helping plasmids needed for virus
packaging are transferred to 293T cells to produce lentivirus particles needed for cell
transduction.
2) Lentivirus
particles infect target cells, and lentivirus surface envelope protein combined with cell
receptors to get into cells.
3) Lentivirus
RNA is reverse transcribed into double-stranded DNA.
4) Double-stranded
DNA and integrase form a pre-integration complex, and then enter the nucleus through the nuclear
pore.
5) Integrase
integrates LTR-flanked sequence into cell genome.
6)
The integrated foreign DNA starts to transcribe and express the target gene.
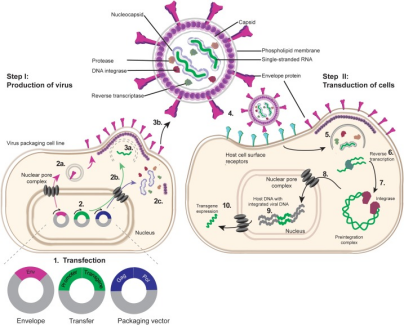
Figure
3. Lentivirus production and transduction process [3]
After
knowing the principles
of the three transfection methods, here
we summarized
their advantages and disadvantages.
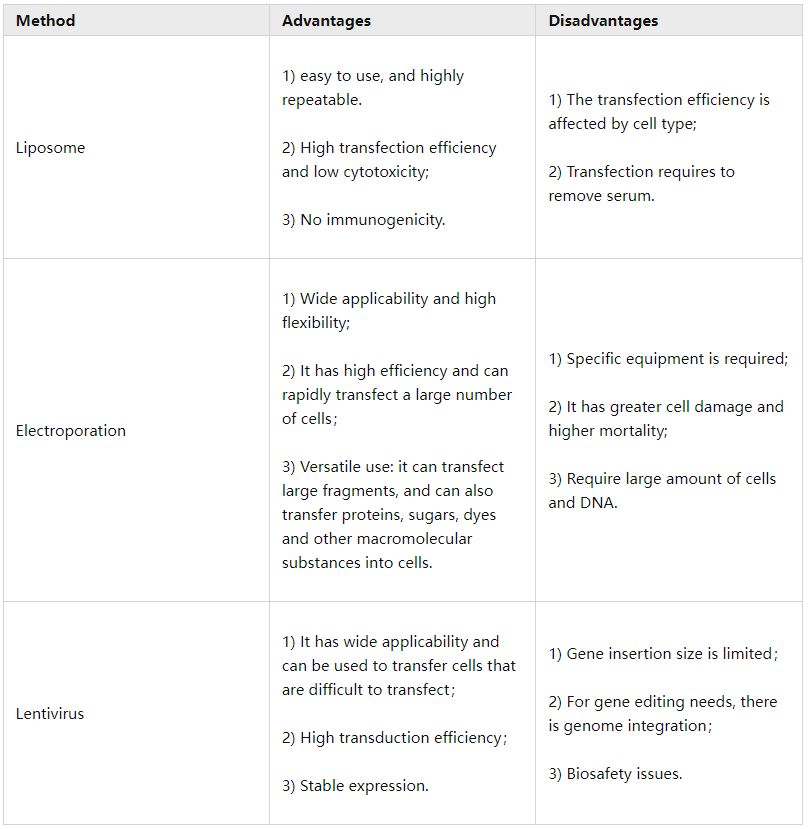
So
how can you find out which transfection method is best for your experiment?
1. Understanding
the cell line: First,
we need to sort out the cell background, the source, cell characteristics, culture conditions,
current passage, subculture period and ratio, etc.
2.
Reference transfection cases: finding
successful transfection cases for reference can help you avoid detours! On the one hand, you can
communicate with the transfection reagent supplier or electroporation instrument supplier to ask
the efficiency and parameters for a certain cell line. On the other hand, you can search for the
literature to check for the methods used in this cell line, and the detailed process. In
addition, it is preferred to choose the transfection method according to the existing
instruments and reagents of the laboratory, and perform efficiency tests.
3.
Preliminary tests: With
the above preparations, we can start the preliminary experiments to find out the best
transfection parameters. Generally, the carrier with fluorescent label is used to test the
transfection rate. Carry out transfection test on cells according to the data you get from the
above step or the transfection protocol provided by the supplier. During the test, generally
measure multiple groups of experimental parameters (such as different ratios of DNA and
transfection reagents by liposome method, different parameters by electroporation, and different
MOI by lentivirus method). Observe the transfection rate 24h to 48h after transfection, and
select the group with the highest efficiency and good cell status for subsequent formal
experiments. If the transfection efficiency of the highest group is less than 30%, it indicates
that this method is not applicable to this cell line, and it is recommended to replace other
transfection methods.
After
reading the above steps, do you want to try it yourself? Ubigene provides in-stock
lentivirus and transfection
medium to
help you test the transfection efficiency!
If
you think it is time-consuming and laborious to explore the transfection parameters in the
preliminary tests, you can also choose to hand over the tedious and repetitive cell construction
work to Ubigene. We have rich experience in cell engineering and gene editing!
Ubigene
is sophisticated in all kinds of transfection methods. Not only good at the methods mentioned
above, but also master other transfection methods such as adenovirus (ADV), adeno-associated
virus (AAV), and has established a database of more than 200 commonly used cell lines, including
the data of transfection parameters, single-cell clone formation, and antibiotic screening. For
the cell line we did not work with, we have a mature procedure to find out the best method to
generate the customized cell line.
With
the help of the transfection culture medium, we can achieve efficient transfection in some
difficult-to-transfect cells, such as THP-1, RAW264.7, MDA MB-231, iPS/ES, and lay a good
foundation for the follow-up work of stable cell line construction!
Custom
KO, KI and overexpression cell lines, deliver as fast
as 4
weeks! Discuss
your project with our specialist NOW>>
Related
products:
Transfection
culture medium>>Increase
transfection efficiency to 80%, reduce cost to a great extent.
In-stock
lentivirus>>Deliver
in 1 week, high titer, high transduction efficiency
Lentivirus
packaging service>>Customized
high titer lentivirus only takes 3 weeks, speed up your cell line
engineering.
3000+
in-stock KO cell lines as low as $1780>>
Over
200 cell lines, thousands of genes, homo-KO clones deliver in 1 week!
200+
in-stock Cas9, luciferase, and EGFP stable cell
lines>>
Expression
validated, facilitate gene-editing cell generation, cell tracking, and in-vivo
imaging.
Shorter
the turnaround of gene-editing cell line to 2-4 weeks--Monoclone Validation
Kit>>
Validate the
clones in early stage, only 15 minutes, 10kb sequence is also feasible.
Reference:
[1]
https://www.mirusbio.com/transfection
[2] Du
X, Wang J, Zhou Q, Zhang L, Wang S, Zhang Z, Yao C. Advanced physical techniques for gene
delivery based on membrane perforation. Drug Deliv. 2018 Nov;25(1):1516-1525. doi:
10.1080/10717544.2018.1480674. PMID: 29968512; PMCID: PMC6058615.
[3] Maes
M E, Colombo G, Schulz R, et al. Targeting microglia with lentivirus and AAV: Recent advances and
remaining challenges[J]. Neuroscience letters, 2019, 707: 134310.










