Published on: November 18, 2024
Views:
--
Characteristics and Applications of the KGN Cell Line
The KGN cell line is an immortalized cell line derived from human ovarian granulosa cells, initially established by Nishi et al.in 1999[1]. Due to its ability to mimic the biological characteristics of granulosa cells, the KGN cell line has been widely used in the study of ovarian physiological functions and related diseases. This article introduces the main characteristics of the KGN cell line and its applications.
How does it differ from other ovarian tumor cell lines?
Compared to other common ovarian tumor cell lines such as OVCAR-3 and SKOV-3, the KGN cell line differs mainly in its origin and physiological functions:
1. Cell origin
The KGN cell line is derived from human ovarian granulosa cell tumors. Granulosa cells are important cell types in the ovary that support follicular development and regulate hormone secretion.
2.Hormonal response
KGN cells exhibits a response to follicle-stimulating hormone (FSH) and expresses functional FSH receptors (FSHR). Under stimulation by FSH and human menopausal gonadotropin (HMG), the activity of aromatase increases, leading to the secretion of estrogen and other hormones.
3.Proliferation and differentiation
KGN cells have a slower doubling time (46.4 hours), which is typical of granulosa cell tumors, and can maintain a differentiated state in vitro.
Therefore, the KGN cell line is widely used to study mechanisms related to follicular development, hormone regulation, granulosa cell tumors, and related diseases (such as polycystic ovary syndrome, PCOS). It serves as an ideal model for studying reproductive disorders and related therapies [2].
Applications of the KGN cell line
1.Ovarian function research
Yang et al. used KGN cells to investigate the impact of autophagy on hormone secretion as the in vitro validation. The inhibition of 3-methyladenine (3-MA) to autophagy initiation promoted smooth autophagic flux and partially reduced the secretion of estradiol and progesterone from KGN cells. In contrast, rapamycin (RAPA), which blocks autophagic flux, further increased the secretion of estradiol and progesterone. The results suggest that autophagy may affect cholesterol transport in the ovary, indicating that autophagic dysfunction could contribute to abnormal hormone secretion and follicular atresia [3].
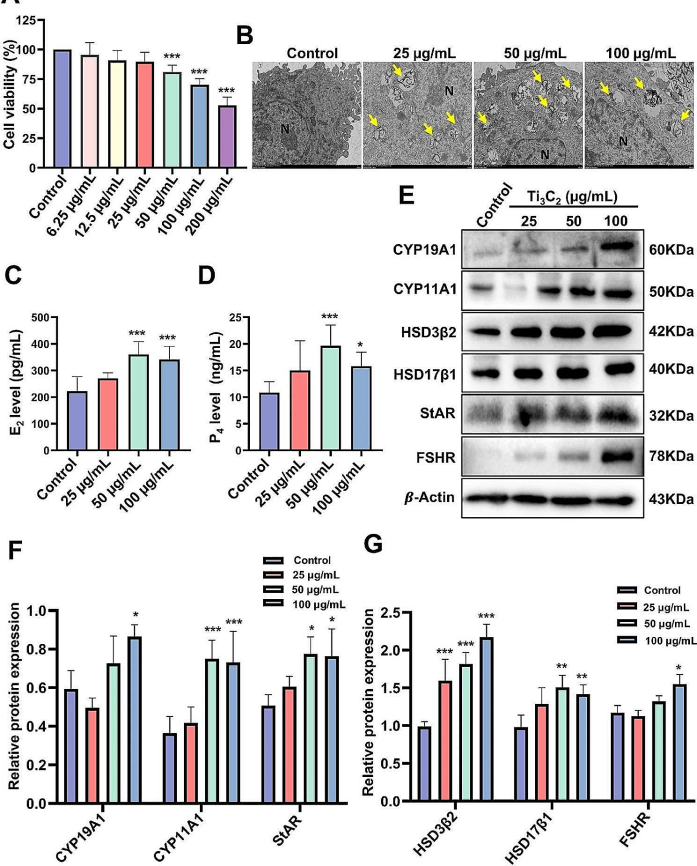
Figure 1. Effect of Ti3C2 nanosheet exposure on the viability and hormone secretion of KGN cells
2.Signal pathway research
Wang et al.established FUT8-knockdown (KGN-KD) and FUT8-restored (KGN-KD-Re) cells based on the KGN cell line.They found that FUT8 plays an important role in core fucosylation, cell proliferation, and signaling pathways regulated by FSHR by examining core fucosylation levels, FUT8 enzyme activity, FUT8 mRNA expression, cell proliferation, FSHR expression and related signaling pathways etc [4].
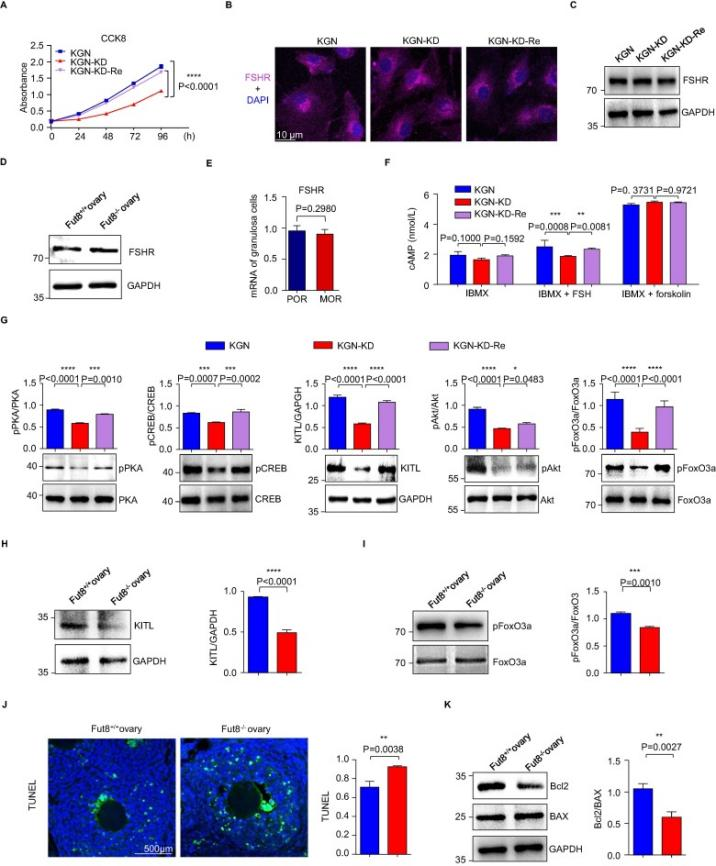
Figure 2. FUT8 knockdown attenuates the FSH/FSHR signaling pathway
3.Reproductive disease target research
In studies on diseases such as polycystic ovary syndrome (PCOS) and ovarian cancer, KGN cells provide valuable tools for uncovering the molecular mechanisms of these diseases. Tan et al. transfected KGN cells with si-NC, si-miR93-5p, oe-NC, and oe-miR93-5p to study the function and regulatory mechanisms of granulosa cells (GCs). They found that miR-93-5p promotes GC apoptosis and ferroptosis by regulating the NF-κB signaling pathway, ultimately identifying miR-93-5p as a potential new molecular target for improving GCs function in PCOS patients [5].
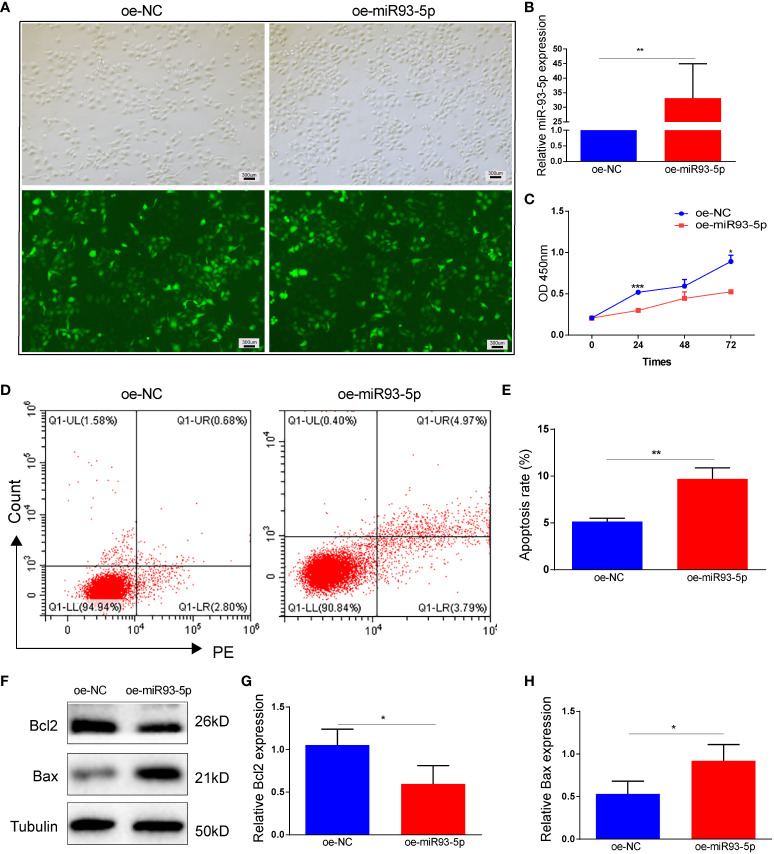
Figure 3. Effect of overexpression of miR-93-5p on apoptosis of KGN cells.
4.Anti-cancer drug development
KGN cells have also been used to study drug targets for treating ovarian granulosa cell tumors (GCTs). Bagnjuk et al. evaluated the expression of cellular inhibitor of apoptosis protein 1 (cIAP1) in granulosa cell tumors and used KGN cells to examine the effects of SMAC mimetic BV-6. The results showed that BV-6 can induce apoptosis in KGN cells, providing a potential therapeutic strategy for GCTs [6].
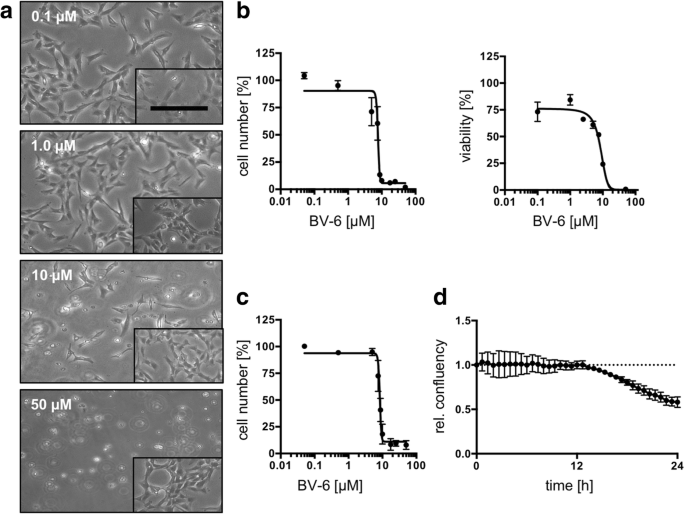
Figure 4. Effect of BV-6 treatment on KGN cells
As a stable granulosa cell model, the KGN cell line has become an indispensable tool in reproductive medicine research. It plays a key role in the study of ovarian function and hormone regulation and provides a foundation for the treatment of female reproductive system diseases.
References:
1.Nishi, Y et al. “Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor.” Endocrinology vol. 142,1 (2001): 437-45. doi:10.1210/endo.142.1.7862
2.Havelock, Jon C et al. “Ovarian granulosa cell lines.” Molecular and cellular endocrinology vol. 228,1-2 (2004): 67-78. doi:10.1016/j.mce.2004.04.018
3.Yang, Limei et al. “Ti3C2 nanosheet-induced autophagy derails ovarian functions.” Journal of nanobiotechnology vol. 22,1 242. 12 May. 2024, doi:10.1186/s12951-024-02495-4
4.Wang, Tiantong et al. “Core fucosylation regulates the ovarian response via FSH receptor during follicular development.” Journal of advanced research, S2090-1232(24)00038-9. 26 Jan. 2024, doi:10.1016/j.jare.2024.01.025
5.Tan, Wei et al. “MiR-93-5p promotes granulosa cell apoptosis and ferroptosis by the NF-kB signaling pathway in polycystic ovary syndrome.” Frontiers in immunology vol. 13 967151. 19 Oct. 2022, doi:10.3389/fimmu.2022.967151
6.Bagnjuk, Konstantin et al. “Inhibitor of apoptosis proteins are potential targets for treatment of granulosa cell tumors - implications from studies in KGN.” Journal of ovarian research vol. 12,1 76. 14 Aug. 2019, doi:10.1186/s13048-019-0549-6