
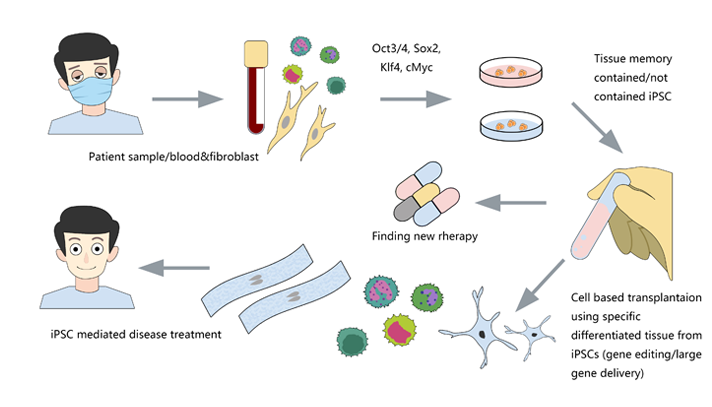
Many serious diseases cannot be cured by medicines, such as heart failure, Late Stage
Diabetes, hemophilia, myeloma, End-Stage Cirrhosis, etc. The best method is allogeneic
transplantation. However, due to the limited donors and the risk of immune rejection,
researchers are dedicated to finding more efficient and safer treatment besides allogeneic
transplantation. Induced pluripotent stem cells (iPSCs) can be derived from the body cells
of the patients themselves, which eliminates the risk of immune rejection, and has the
potential of differentiation into different cells. Transplantation of cells derived from
iPSC, such as cardiomyocytes, hepatocytes, neurocytes, T cells, hematopoietic stem cells
(HSCs) and pancreatic cells, is possible to solve many medical problems.
Hepatocyte
The differentiation of liver cells induced by iPSC can alleviate the shortage of
sources in liver transplantation and hepatocyte transplantation, which is more
conducive to basic and clinical research. In addition, the induced hepatocyte
could
be used as a tool to simulate and study liver diseases and screen the
hepatotoxicity
of drugs in the future.
Neural stem cell and neuron
Neural stem cells differentiated from iPSC can be used to generate cell models
of
nervous system diseases. This approach avoids ethical problems and immune
rejection,
and is an ideal way to obtain NSC in vitro.
iPSC can differentiate into neuron under appropriate conditions. For example, differentiation into motor neurons (MN) provides the possibility for the treatment and research of MN injury diseases such as Amyotrophic lateral sclerosis (ALS) and Spinal muscular atrophy (SMA).
iPSC can differentiate into neuron under appropriate conditions. For example, differentiation into motor neurons (MN) provides the possibility for the treatment and research of MN injury diseases such as Amyotrophic lateral sclerosis (ALS) and Spinal muscular atrophy (SMA).
T cell
iPSC can differentiate into T cell. The CAR-T cell therapy developed on the
basis of
iPSC has a safer and more effective pharmacological activity. iPSCs based CAR-T
cells can be used in T cell immunotherapy without the limitation of Allograft
rejection.
Hematopoietic stem cell
The limited number of hematopoietic stem cells (HSC), the difficulty of
expansion
and culture in vitro and graft versus host disease (GVHD) limit the HSC
transplantation. iPSC can proliferate and differentiate into transplantable HSCs
in
vitro, which brings a bright future for the treatment of malignant blood
diseases.
Cardiomyocyte
iPSC derived cardiomyocytes provide a new way for the study of disease-specific
and
individual-specific pathogenesis of cardiovascular diseases, which has become an
effective tool in the field of cardiovascular research and also brings new hope
for
clinical treatment.
Pancreatic cell
iPSC can differentiate into pancreatic β-cells in vitro, which can be used in
the
research of disease mechanism, drug development and cell therapy for diabetes.
Using
this source of pancreatic β-cells for transplantation in the treatment of
diabetes
can better solve the ethical, limited source problems faced by the previous
islet
transplantation.
Ubigene’s iPSC platform:
Reprogramming services
By transferring transcription factors, such as
Oct3/4、Sox2、c-Myc
and KlF4, somatic cells could be reprogrammed into iPSC with the potential of
proliferation and differentiation.
Steps of iPSC reprogramming:
· Vectors
carrying
transcription factors will be transferred into somatic cells to reprogram into
iPSC;
· iPSC
validation:
genotyping and phenotyping.
Gene editing service
The success rate of gene editing in human iPSC is lower because, unlike tumor
cell
lines, iPSC does not have the characteristics of chromosomal abnormality and
strong
ability of DNA repair. CRISPR/Cas9 has the advantages of high efficiency, easy
to
construct and low toxicity in human cells, so it is the most common method in
iPSC
genome editing. CRISPR-U™ optimizes the targeting efficiency, greatly improve
the
efficiency of DSB and homologous recombination in iPSCs.
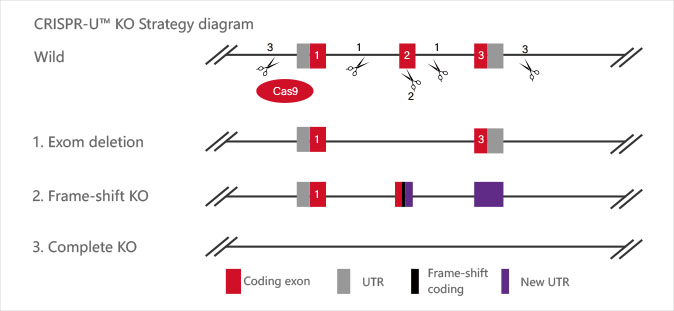
Knockout
CRISPR-U™ gene knockout iPSC cell line: gRNA and Cas9 are
transferred
into iPSCs by nucleofection. After drug screening, single clones would
be
generated. Positive clones would be validated by sequencing.
| Type | Strategy | Application |
| Short fragment removal | Guide RNAs target introns at both sides of exon 2 and the number of bases in exon 2 is not a multiple of 3, which can cause frame-shift mutation. | Study of gene function through gene defect |
| Frame-shift mutation | Guide RNA targets the exon, and the base number of deletion is not a multiple of 3. After knockout, frame-shift mutation would cause gene knockout. | |
| Large fragment removal | Complete removal of the coding sequence to achieve gene knockout. |
Case Study:
The limited T cells and the difficulty of
proliferation is
the main obstacle of T-cell immunotherapy, which can be overcome by
using
pluripotent stem cells with proliferation and differentiation ability to
generate T-iPSC with antigen specificity. Strict antigen specificity is
essential for safe and effective T-cell immunotherapy. However, in the
process of double-positive CD4/CD8 differentiation, the rearrangement of
the
T-cell receptor (TCR) α chain will lose antigen specificity. This TCR
rearrangement was prevented by removing the recombinant enzyme gene
(RAG2)
in T-iPSCs with CRISPR/Cas9. Xenotransplantation of CD8αβ-T cells with
stable TCR can effectively inhibit tumor growth in disease models. This
contributes to a safe and effective T-cell immunotherapy.
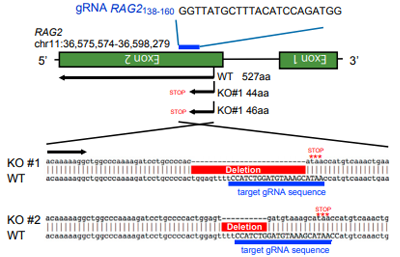
gRNA sequence and RAG2ockout sequence。The positive
clones
have frameshift mutations in the designated RAG2.

Comparison of the binding ability of WT and RAG2
knockout
T-iPSCs to dextramer. RAG2-/- T-iPSCs differentiated into CD8αβ cells
expressing stable TCR, while 40% of RAG2wt/wt-iPSC derived CD8αβ cells
lost
antigen specificity.
Reference:
Minagawa, Atsutaka, et al. "Enhancing T cell receptor stability in
rejuvenated iPSC-derived T cells improves their use in cancer
immunotherapy." Cell Stem Cell 23.6 (2018): 850-858.
Gene modeling or repair
Point Mutation
iPSC would be co-transfected with gRNA, Cas9 and donor oligo by
electroporation. After the DNA DSB caused by the complex of gRNA and
Cas9,
iPSCs use donor oligo carrying wild-type sequence as a template for
homologous recombination repair (HDR) and replace the target sequence
with
point mutation.
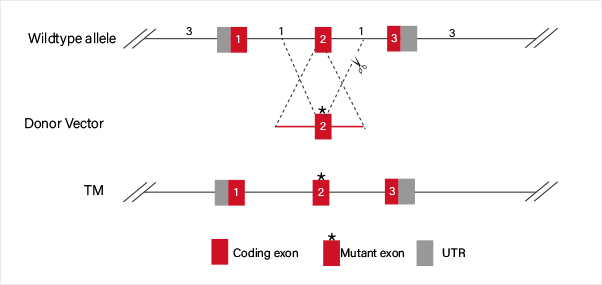
Case Study:
ssODN carrying point mutation which replaces the WT sequence by HDR.
ssODN carrying WT sequence which replaces the mutated site by HDR.
The limited T cells and the difficulty of
proliferation is
the main obstacle of T-cell immunotherapy, which can be overcome by
using
pluripotent stem cells with proliferation and differentiation ability to
generate T-iPSC with antigen specificity. Strict antigen specificity is
essential for safe and effective T-cell immunotherapy. However, in the
process of double-positive CD4/CD8 differentiation, the rearrangement of
the
T-cell receptor (TCR) α chain will lose antigen specificity. This TCR
rearrangement was prevented by removing the recombinant enzyme gene
(RAG2)
in T-iPSCs with CRISPR/Cas9. Xenotransplantation of CD8αβ-T cells with
stable TCR can effectively inhibit tumor growth in disease models. This
contributes to a safe and effective T-cell immunotherapy.

CRISPR/Cas9 and ssODN used to repair the point
mutation in
A79V-hiPSC. A) Genomic sequence surrounding the mutation site: mutated
nucleotide (T, red); sgRNA recognition site containing 20 bp (yellow);
CRISPR cutting site between the 17th and 18th bp (bold); forward and
reverse
primers (pink). B) ssODN with 120 bp, 60 bp upstream and 60 bp
downstream
the mutation site containing the WT nucleotide (C, green).
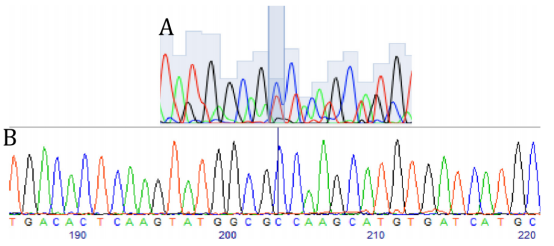
Sequencing of exon 4 of the PSEN1 gene in hiPSCs.
A) Heterozygous c.236C>T substitution in the mother line previously published.
B) Successful correction of the point mutation (T>C).
A) Heterozygous c.236C>T substitution in the mother line previously published.
B) Successful correction of the point mutation (T>C).
Reference:
Pires, C., Schmid, B., Petræus, C., Poon, A., Nimsanor, N., Nielsen, T.
T.,
... & Freude, K. K. (2016). Generation of a gene-corrected isogenic
control
cell line from an Alzheimer's disease patient iPSC line carrying a A79V
mutation in PSEN1. Stem cell research, 17(2), 285-288.
Gene Knock in
Knock in
CRISPR-U™ Gene Knockin iPSC:iPSC would be co-transfected with
gRNA, Cas9 and donor vector by electroporation. After drug
screening,
single clones would be generated. Positive clones would be validated
by
sequencing.
Knockin Strategies :
Disease model generation
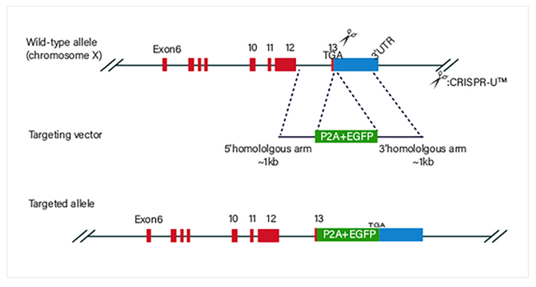
Guide RNA and Cas9 complex cause a double-strand break (DSB) on the
target site of DNA. The donor vector carrying knockin sequence is
the
template for homologous recombination repair (HDR), and it
recombines to
the target site.
Safe harbor knockin:
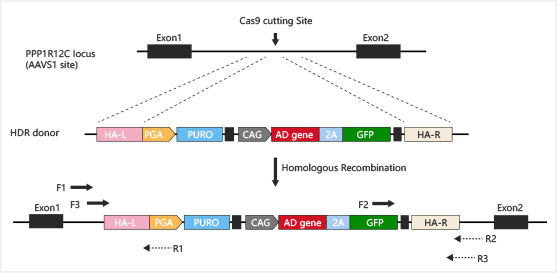
Gene knockin at Safe harbors such as hROSA26 and AAVS1 not only
avoids
random insertion in genome, but also achieves overexpression of
target
gene.
Case Study:
The most common method to treat hemophilia is substitution therapy,
but
this method has the risk of virus infection, and it is a method that
needs lifelong continuous treatment. Gene therapy seems like the
only
way can cure hemophilia. CRISPR/Cas9 technology can be used for gene
therapy of hemophilia. The mutations of coagulation factors, F8 and
F9,
are the main causes of hemophilia. Previous studies have shown that
F9
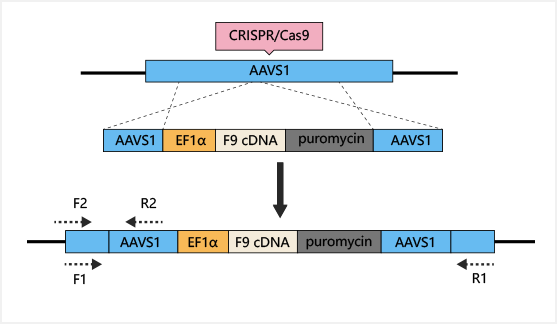
is a more effective gene therapy target. AAVS1-Cas9-sgRNA plasmid
and
AAVS1-EF1α-F9 cDNA puromycin donor plasmid were constructed and
transferred into iPSC. Human factor IX (hFIX) antigen activity was
detected in the culture supernatant. Finally, liver cells
differentiated
from iPSC were transplanted into NOD/SCID mice by spleen injection,
to
cure hemophilia B.
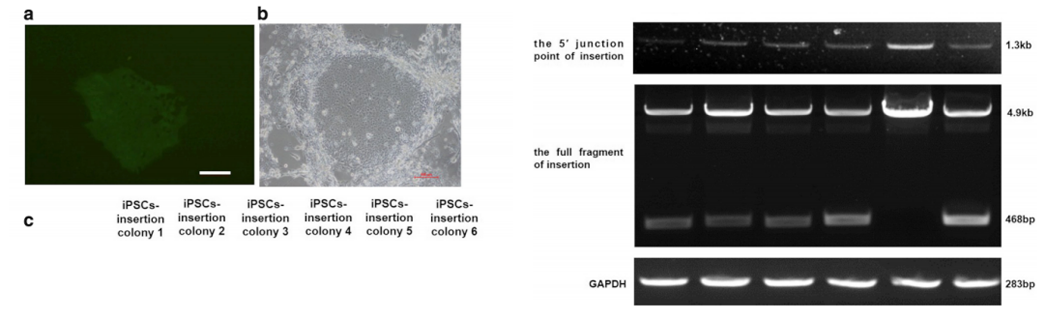
After 48 hours of transfection, puromycin was used for drug
screening.
Most iPSCs died after drug screening, but a few survived. After
about 7
days, each surviving iPSC clone was expended to be further testing
of
insertion (Fig. a, b). Six clones were selected. As shown in Figure
C,
1.3kb fragments can be detected in all iPSC clones with primers;
468bp
and 4.9kb fragments can be detected in iPSC clones 1, 2, 3, 4 and 6
with
another pair of primers, indicating F9 cDNA heterozygous insertion;
only
4.9kb fragments can be detected in iPSC clone 5, indicating F9 cDNA
homozygous insertion.
Reference:
Lyu, Cuicui, et al. "Targeted genome engineering in human induced
pluripotent stem cells from patients with hemophilia B using the
CRISPR-Cas9 system." Stem cell research & therapy 9.1 (2018): 92.
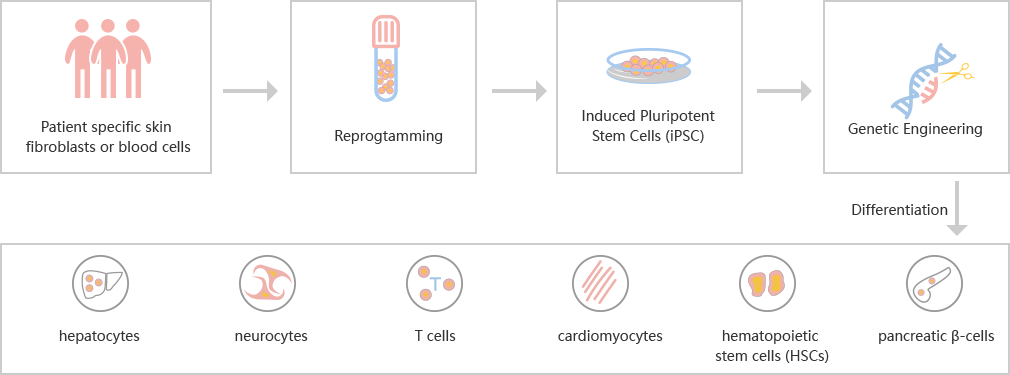
iPSC differentiation
The study of human embryonic stem cells (hESCs) derived from
early embryos has been controversial in ethics, and the rejection of
differentiated
cells derived from hESCs in transplantation has limited its clinical
application.
Hepatocytes, nerve cells, T cells, cardiomyocytes, hematopoietic stem cells and
islet cells can be differentiated from patients' somatic cells (such as
fibroblasts)
or existing iPSCs.
Differentiation
Process






