CRISPR
Library Screening Applications in Research on Acute Leukemia
Introduction of Acute Leukemia
Acute leukemia can be divided into two types, acute myeloid
leukemia (AML) and acute lymphoblastic leukemia (ALL), and is one of
the most
common blood tumors in adults. Its onset is due to the malignant proliferation of hematopoietic stem cells and
the
loss of their ability to differentiate normally, inhibiting the normal function of hematopoietic cells, which
results in severe consequences such as anemia, bleeding, and immunosuppression. In recent years, with the
development of CRISPR technology, especially the application of CRISPR library screening, a large number
of
targets for acute leukemia-related treatments have been discovered. Various new types of targeted
drugs such as IDH1/2 inhibitors, FLT3 inhibitors, BCL-2 inhibitors, and CPX-351 have gradually
entered
clinical trials, leading to a major shift in acute leukemia treatment from chemotherapy to targeted
therapy[1-2]. So how is CRISPR library screening applied in the research of acute leukemia? Today
Ubigene
has compiled several high-score papers, let’s learn from these paper and find out the answers.
Application of CRISPR Library Screening in AML
Research
1.Finding Genes Necessary for AML Cell Growth
For CRISPR library screening, the most direct application is to
screen for genes necessary for cell growth. However, to ensure the reliability of the screening results, it is
best
to use multiple different cell lines and combine in vivo models for synchronous screening. Lin et al. selected
200
growth-dependent genes in AML cell lines from the database, and then constructed the corresponding AML cell growth-dependent gene knockout libraries. By using
these
libraries for screening two different AML cell lines, MV4-11 and U937, as
well
as a tumor xenograft model PDX (patient-derived xenograft), the researchers found that the SLC5A3 and MARCH5
genes
were significantly down-regulated in all three library screening models (Figure 1). Further downstream mechanism
studies by constructing AML cell lines in which these two genes were individually knocked out revealed that
SLC5A3
supports the proliferation of AML cells via myo-inositol transport, while the MARCH5 gene prevents AML cells
from
undergoing apoptosis[3].
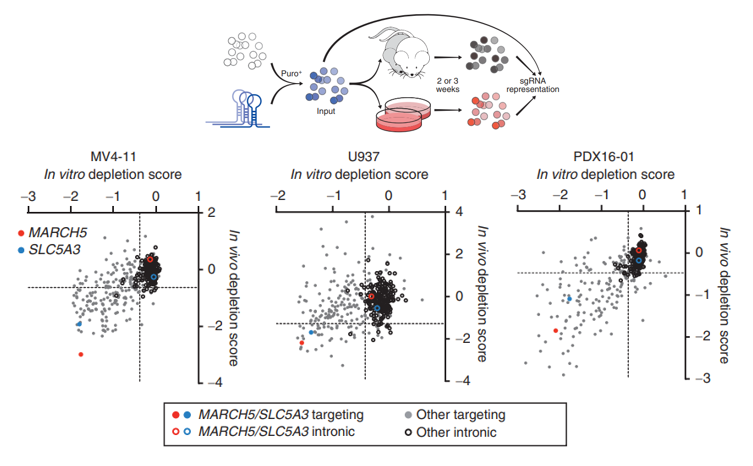
Figure 1 The MARCH5 and
SLC5A3 genes were significantly
down-regulated in CRISPR library screening[3]
2.Promotion of Immune Escape in AML Cells by SUSD6 Gene
In addition to screen for genes necessary for cell growth, using
CRISPR library screening to identify drug-resistant genes is also a common method. Immune checkpoint therapy is
a
method of promoting anti-tumor immune responses by blocking inhibitory signals for T-cell activation. However,
certain tumors exhibit primary resistance or develop adaptive resistance to immune checkpoint therapy, rendering
it
ineffective for the treatment of these types of tumors. To investigate the mechanisms of tumor cell resistance
to
immune checkpoint therapy, a research team focused on human AML cell line
THP-1 and a mouse AML cell line RN2, and used a whole-genome knockout library combined with flow cytometry
to
screen for fluorescently labeled cells expressing high levels of tumor antigens and found significant enrichment
of
the SUSD6 gene in both AML cell lines (Figure 2). This suggests that
knockout of the SUSD6 gene promotes the
expression of tumor antigens, enhancing the efficacy of immune checkpoint therapy. Subsequent experiments
involving
SUSD6 gene knockout in cells and in vivo experiments confirmed that SUSD6 inhibits MHC-I expression in AML cells
and
its immunogenicity to promote immune escape responses in cells[4].
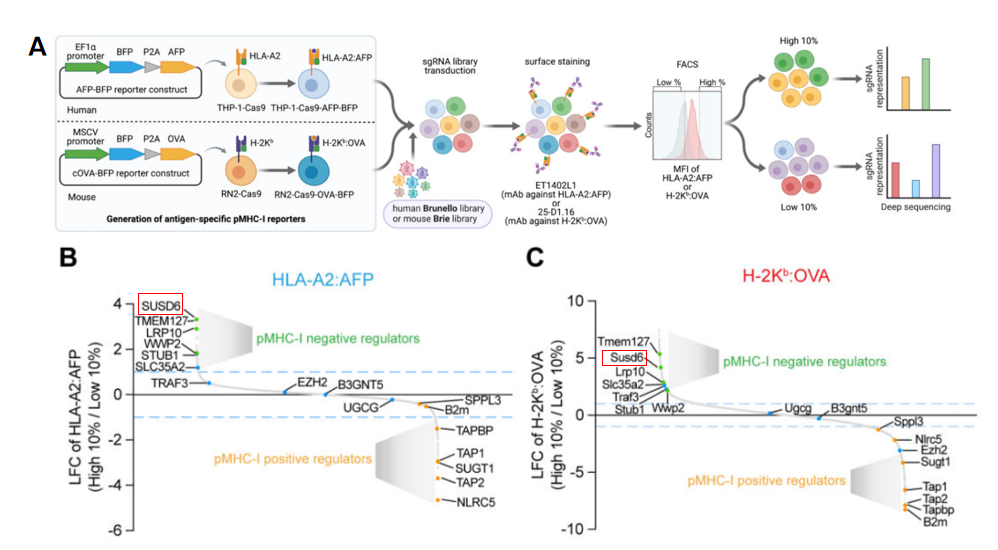
Figure
2 The SUSD6 gene was
enriched in cell populations expressing high levels of tumor antigens in two cell
lines[4]
Application of CRISPR Library Screening in ALL Research
Acute lymphoblastic leukemia can be further divided into two types:
acute T lymphoblastic leukemia (T-ALL) and acute B lymphoblastic leukemia (B-ALL), which account for
approximately
30% of acute leukemia. Although the incidence rate of ALL is lower than AML, compared with AML, ALL is more
difficult to treat and has a poor prognosis. Currently, there is no FDA-approved treatment for ALL.
1.Significant Upregulation of Valine-tRNA Synthetase
in T-ALL
tRNA biosynthesis disturbances are common in tumor cells; however,
it is unknown whether tRNA biosynthesis disturbances occur in T-ALL. To investigate this issue, Thandapani et
al.
used a knockout library of tRNA biosynthesis-related genes to screen two different
T-ALL cell lines, CUTLL1 and Jurkat, and found that the gene
VARS,
responsible for valine-tRNA synthesis, was significantly downregulated in these two cell lines (Figure 3).
Analysis
of T-ALL samples revealed significant upregulation of VARS mRNA expression. These results suggest that
valine-tRNA
biosynthesis is disrupted in T-ALL. To further validate the role of valine-tRNA biosynthesis, the researchers
found
that the knockout of VARS gene knockouts in cell lines or restricting valine
intake of
animals can significantly inhibit T-ALL proliferation and improve T-ALL symptoms (Figure
4)[5].
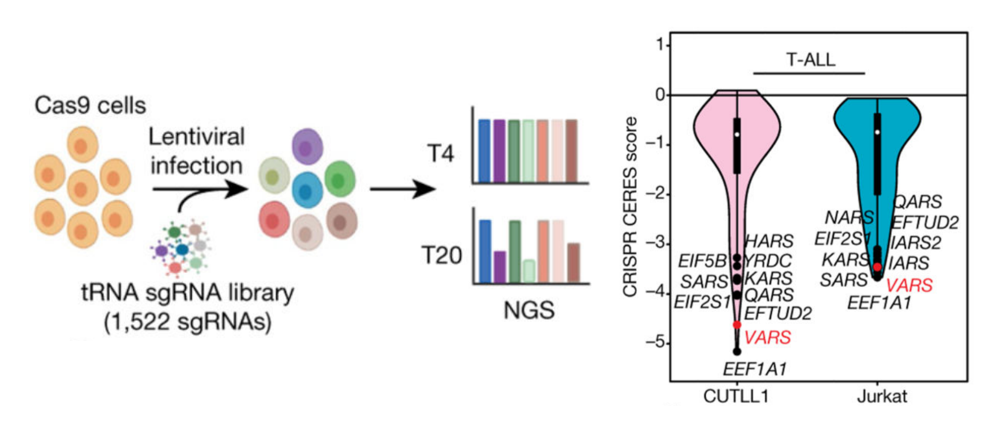
Figure
3 The VARS gene was
significantly downregulated in CRISPR library screening[5]
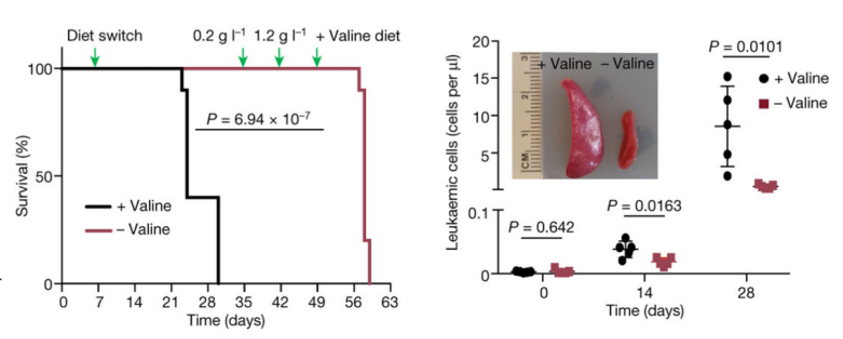
Figure
4 Restricting valine intake
significantly improved T-ALL mouse symptoms[5]
2.NUDT21 Gene Negatively Regulates CD19 Expression in
B-ALL
Lack of CD19 expression in B-ALL cells leads to
resistance to CAR-T cell therapy, but how CD19 expression is regulated remains unknown. To explore this issue,
Witkowski et al. used a whole-genome knockout library to
screen three different B-ALL cell lines, Reh, 697, and NALM6. After
performing flow
cytometry to select for cell populations expressing high and low levels of CD19 and performing NGS
sequencing analysis, NUDT21 was found to be significantly enriched in cell populations expressing
high
levels of CD19 in the three cell lines. It indicates that NUDT21 negatively regulates CD19 expression (Figure
5).
After confirming high expression of NUDT21 in B-ALL, the research team used the CRISPR/Cas9 system to construct
NUDT21 gene knockout B-ALL cell lines and found that NUDT21 directly inhibits the stability and protein
expression
of CD19 mRNA[6].
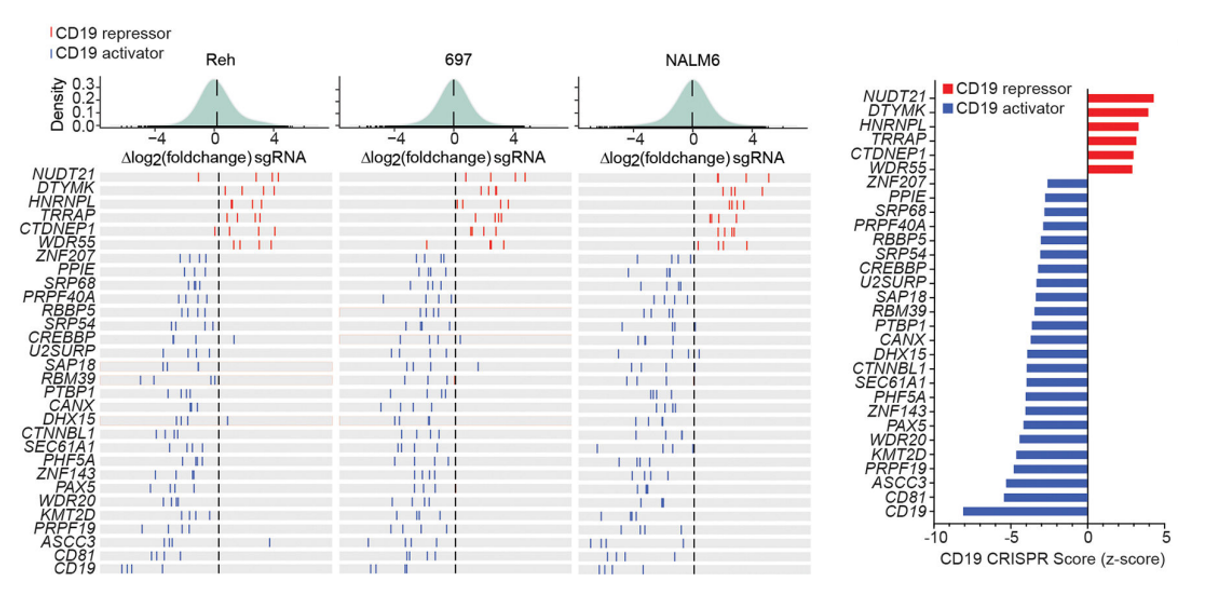
Figure 5 NUDT21 was
significantly enriched as a CD19
inhibitor[6]
Conclusion
The development of CRISPR library
screening technology has been ongoing for 10 years, and with the help of CRISPR library screening, numerous
novel
targets have been discovered in different fields, leading to the publication of many high-impact articles. Based
on
the above cases, it is easy to see that in recent years, high-impact articles published using CRISPR library
screening generally have the following features in common: 1. Using multiple different cell lines for synchronous
screening of
a specific disease, and identifying targets through comparative analysis; 2. After identifying targets, constructing gene knockout cell lines for
validation and
conducting downstream mechanism studies. In addition, using smaller sgRNA libraries, combining flow cytometry
sorting, and animal experiments are more likely to gain favor with reviewers for your research.
In order to help researchers conduct better research and publish
high-quality articles, we have compiled a list of various cell lines commonly used in acute leukemia (Table 1)
for
your convenience. If you have research needs in acute leukemia, be sure to bookmark this information!
Table 1 Commonly Used Cell Lines in Acute Leukemia
Leukemia Type | Species | Common Cell
Lines |
AML | Human | THP-1*,
MV4-11, U937, OCI-AML5, MOML13, MOML14,
HL-60 |
Mouse | RN2,
C1498,
EL9611, RAW264.7* |
T-ALL | Human | Jurkat*,
CUTTL1, Hut78, JM, CCRF-CEM |
|
B-ALL | Human | NALM-6, REH,
697, BALL-1, EHEB |
|
* Ubigene
provides CRISPR library
screening services for popular
cell lines such as THP-1, RAW264.7, Jurkat, etc. After identifying targets, you can validate them using
Ubigene's
cell bank, containing 4500+ KO cell lines, in which 300+ selected genes enjoy a special price of
only
$1780.
Contact us for more details!
References
[1] Lin T, Liu D, Guan Z, Zhao X, Li S, Wang X, Hou R, Zheng J, Cao J, Shi M. CRISPR screens
in mechanism and target discovery for AML. Heliyon. 2024 Apr 9;10(8):e29382.
[2] Kayser S, Levis MJ. Updates on targeted therapies for acute myeloid leukaemia. Br J
Haematol. 2022 Jan;196(2):316-328.
[3] Lin S, Larrue C, Scheidegger NK, Seong BKA, Dharia NV, Kuljanin M, Wechsler CS,
Kugener G, Robichaud AL, Conway AS, Mashaka T, Mouche S, Adane B, Ryan JA, Mancias JD, Younger ST,
Piccioni F,
Lee LH, Wunderlich M, Letai A, Tamburini J, Stegmaier K. An In Vivo CRISPR Screening
Platform
for Prioritizing Therapeutic Targets in AML. Cancer Discov. 2022 Feb;12(2):432-449.
[4] Chen X, Lu Q, Zhou H, Liu J, Nadorp B, Lasry A, Sun Z, Lai B, Rona G, Zhang J,
Cammer M, Wang K, Al-Santli W, Ciantra Z, Guo Q, You J, Sengupta D, Boukhris A, Zhang H, Liu C,
Cresswell P,
Dahia PLM, Pagano M, Aifantis I, Wang J. A membrane-associated MHC-I inhibitory axis for cancer immune
evasion.
Cell. 2023 Aug 31;186(18):3903-3920.e21.
[5] Thandapani P, Kloetgen A, Witkowski MT, Glytsou C, Lee AK, Wang E, Wang J, LeBoeuf
SE, Avrampou K, Papagiannakopoulos T, Tsirigos A, Aifantis I. Valine tRNA levels and availability
regulate
complex I assembly in leukaemia. Nature. 2022 Jan;601(7893):428-433.
[6] Witkowski MT, Lee S, Wang E, Lee AK, Talbot A, Ma C, Tsopoulidis N, Brumbaugh J,
Zhao Y, Roberts KG, Hogg SJ, Nomikou S, Ghebrechristos YE, Thandapani P, Mullighan CG, Hochedlinger K,
Chen W,
Abdel-Wahab O, Eyquem J, Aifantis I. NUDT21 limits CD19 levels through alternative mRNA polyadenylation
in B
cell acute lymphoblastic leukemia. Nat Immunol. 2022 Oct;23(10):1424-1432.