KO Cell Line Bank
STR authentication, contamination free, good viability
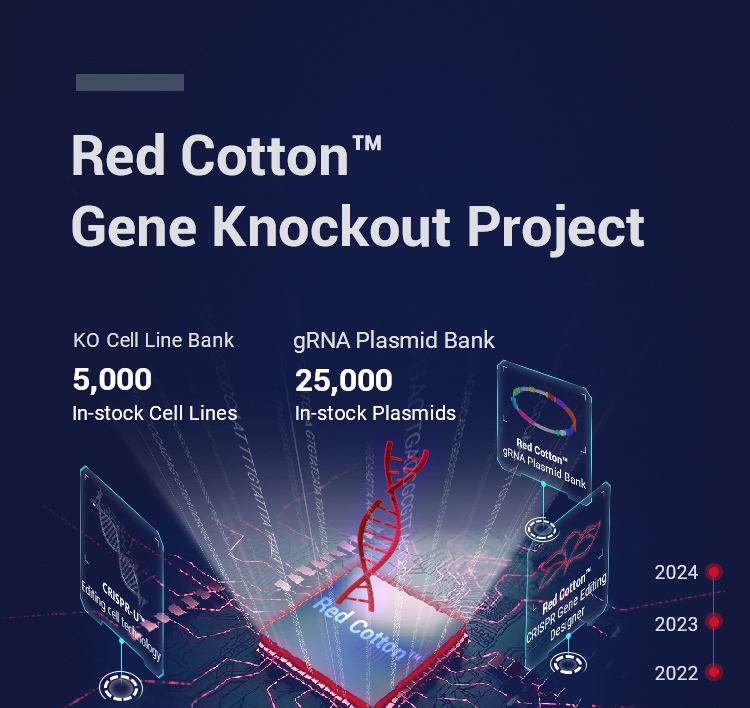
KO Cell Line Bank
STR authentication, contamination free, good viability
 Luciferase Stable Cell Lines
Low passage, good viability
Luciferase Stable Cell Lines
Low passage, good viability
 Cas9 Stable Cell Lines
High editing efficiency, good viability
Cas9 Stable Cell Lines
High editing efficiency, good viability
 Wild-type Cell Lines
STR authentication report available, low passages, high activity and good cell condition
Wild-type Cell Lines
STR authentication report available, low passages, high activity and good cell condition