[Research Highlight] miR-302a is involved in vascular smooth muscle cell proliferation and restenosis
Vascular stenosis in different parts of body can lead to
diseases of organs or tissues dominated by
blood vessels in corresponding areas, such as Acute coronary syndrome(ACS) which is led by coronary artery stenosis and other cardiovascular diseases. ACS is a common serious cardiovascular disease, a serious type of coronary heart disease, percutaneous
coronary
interventions (e.g., balloon angioplasty or stenting) are considered the first-choice treatment for it. However, patients face an
increased risk of restenosis after
surgery. Excessive smooth muscle cell (SMC) proliferation, migration and invasion are considered important contributors to restenosis, which together lead to neointimal hyperplasia. Therefore, inhibition of SMC hyperplasia is an important therapeutic approach for restenosis.
MicroRNAs (miRNAs) are a family of endogenous noncoding small RNAs consisting of 18–22 nucleotides that can regulate gene expression at the post-transcriptional level by pairing with imperfect complementary target sites in the 3’UTR of their target mRNAs. Previous studies have suggested that miRNAs are involved in regulating SMC proliferation and migration. The miR-302a functions as a tumor suppressor by inhibiting Akt-dependent cell proliferation in different cancer cells, indicating that miR-302a may negatively regulate Akt activation. And the lack of Akt has been shown to inhibit VSMC proliferation and migration.
Given the importance of Akt in regulating
cell proliferation, Ying-ying Liu et al from SUN YAT-SEN UNIVERSITY speculated
that miR-302a is involved in Akt-mediated vascular SMC (VSMC) proliferation and restenosis. They published an article “MicroRNA-302a promotes neointimal formation
following carotid artery injury in mice by targeting PHLPP2 thus increasing Akt signaling” in Acta Pharmacologica Sinica. In the article, they used MiR-302 heterozygous mice and MiR-302 conditional KO mice building the carotid artery injury model, to investigated whether and how MicroRNAs regulated vascular SMC proliferation and vascular remodeling following carotid artery injury in mice. The study demonstrate that carotid artery
injury-induced neointimal
formation was remarkably ameliorated in miR-302 heterozygous mice and SMC-specific miR-302
knockout mice. In contrast, delivery of miR-302a adenovirus to the injured carotid artery
enhanced neointimal formation. Upregulation of miR-302a enhanced the proliferation and
migration of mouse aorta SMC (MASMC) in vitro by promoting cell cycle transition, whereas
miR-302a inhibition caused the opposite results.

miR-302a potentiates MASMC proliferation by
increasing Akt activity
miR-302a promoted Akt activation by corporately
decreasing Akt expression and increasing Akt phosphorylation in MASMCs. To further confirm whether Akt
activity is required for the regulatory function of miR-302a in VSMC proliferation, cells were treated with
miR-302a mimics and the Akt inhibitor GSK690693 in the presence of PDGF-BB. Compared with miR-302a
mimics-treated MASMCs, treatment with GSK690693 inhibited cell proliferation and migration, which was
potentiated by miR-302a upregulation. These results suggest that miR-302a regulates VSMC proliferation
dependent on Akt signaling.
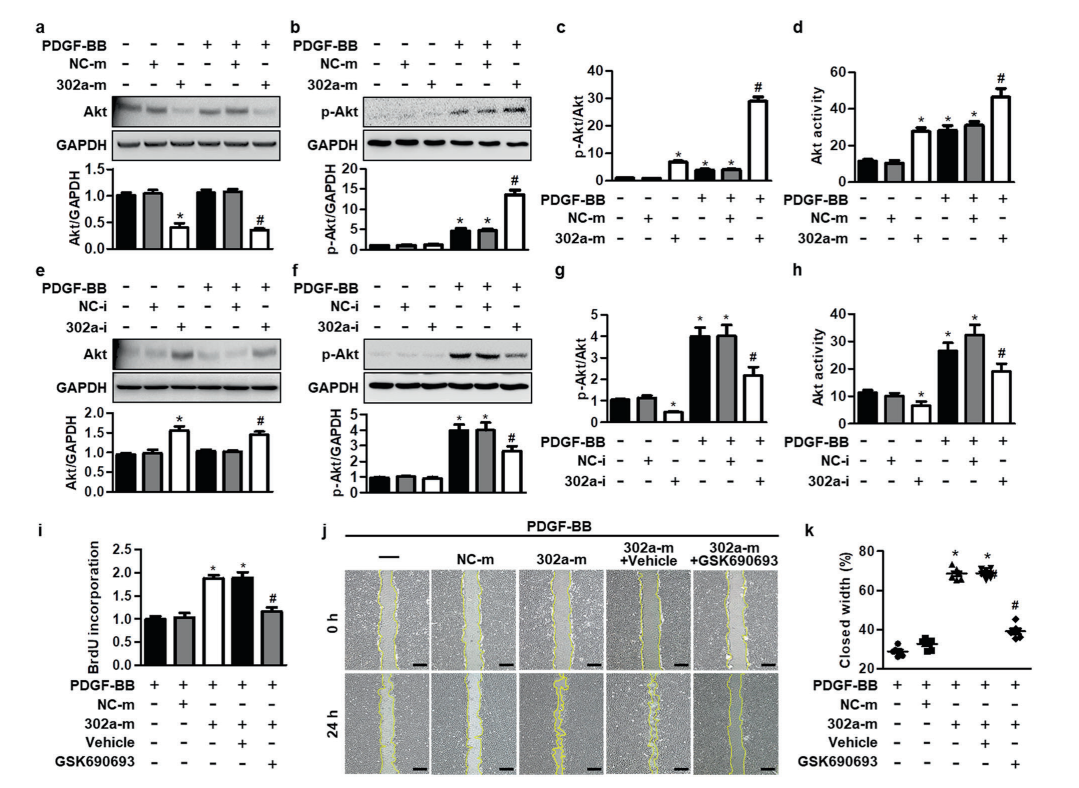
Figure 1
PHLPP2 is essential for the function of miR-302 in neointimal formation
They further revealed that miR-302a directly
targeted at the 3′ untranslated region of PH domain and leucine rich repeat protein phosphatase 2 (PHLPP2) and
negatively regulated PHLPP2 expression. Restoration of PHLPP2 abrogated the effects of miR-302a on Akt activation
and MASMC motility. To further evaluate the requirement of PHLPP2 during miR-302- regulated restenosis in vivo, recombinant adenovirus harboring leucine rich repeat protein phosphatase 2
shRNA (Ad-shPHLPP2) (provided by Ubigene) was administered to miR-302+/− mice. The result showed that the inhibitory effects of miR-302
knockdown on neointimal formation are largely dependent on the elevation of PHLPP2 expression(Fig.2).
The shRNA adenovirus
provided by Ubigene in this study was used to further study the effect of
PHLPP2 on
the formation of mir-302 in neointima. The purity and titer of adenovirus determine its quality and way of use. The
adenovirus particles provided by Ubigene are ultra-centrifuged and filtered, and the titer is determined by
hexonimmunoassay to ensure the purity and accurate titer. At present, Ubigene provides adenovirus
with titer of 10 ^ 10 ~ 10 ^ 12 PFU/ml. Click here to learn >>
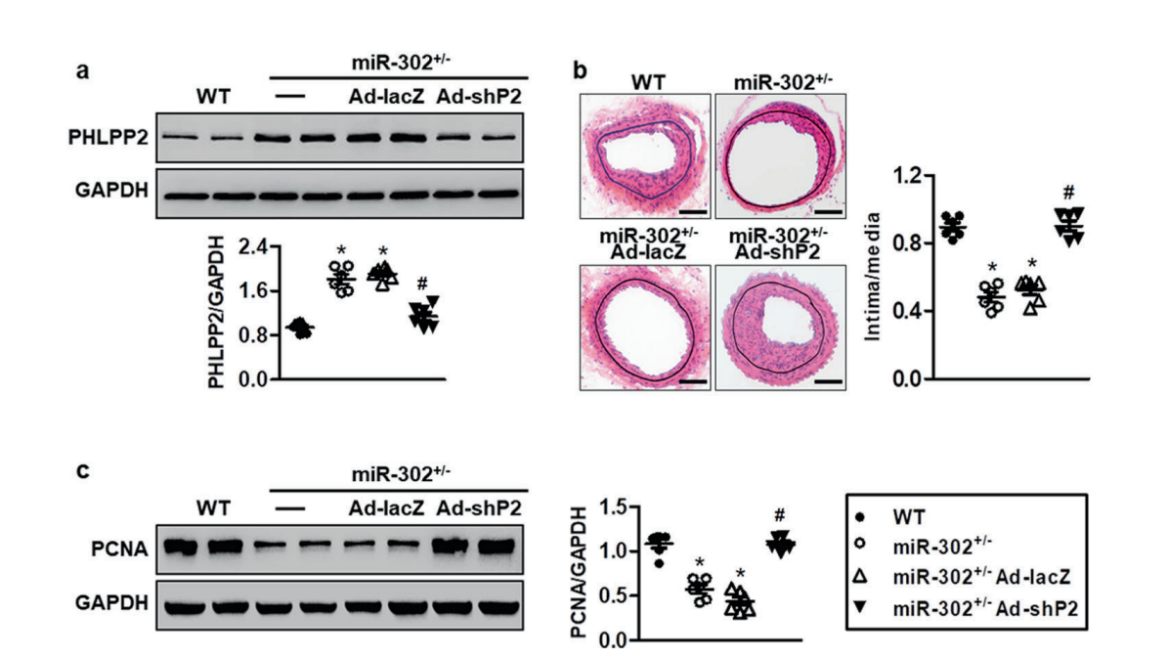
Figure 2
In conclusion, the study demonstrated that miR-302a
potentiates VSMC hyperplasia and subsequent neointimal formation by Akt activation via targeting its
phosphatase PHLPP2, suggesting that inhibition of miR-302a may be a novel strategy for restenosis
treatment, and this study demonstrated for the first time that PHLPP2 is a novel target of miR-302a. It provided a new idea for vascular
restenosis
treatment in future.
Ubigene is focusing on cell genome editing. We provide high-quality gene editing cell lines, stable cell lines, virus packaging and other related services. Our KO cell bank contains nearly 2000 KO cell lines, involving in 8 popular signal pathways, such as key genes of Akt signal pathway. For more details, feel free to contact us!









