Expert Insights | What to Do if RAW 264.7 Cells Differentiate Due to Poor Culturing?

RAW 264.7 Cells (Mouse
Mononuclear Macrophage Leukemia Cells) are derived from male mice induced by the Abelson murine leukemia virus.
Due to their phagocytic and endocytic activities, these adherent cells are considered an ideal in vitro model for
macrophages. RAW 264.7 cells are also used in natural product screening, osteoclast research, microbial immune
modulation, and more. Additionally, gene-editing RAW 264.7 cells can be utilized to validate
inflammatory targets, study macrophage gene functions, etc.
Culturing cells properly is
essential for conducting cell experiments. Those who have cultured RAW 264.7 cells should know that these cells are
very delicate and easily differentiate (develop small protrusions) during culturing. If not addressed
promptly, differentiation can delay the experimental progress. To culture RAW 264.7 cells effectively, every step in
the culture process is crucial. With years of cell culturing experience, Ubigene has prepared the exclusive manual to guide you on how to reduce cell differentiation.
Before jumping into expert insights, let's review some basic
information about RAW 264.7 cells.
Cell Name | RAW 264.7 (Mouse Mononuclear Macrophage Leukemia Cells) |
Growth Characteristics | Adherent |
Cell Morphology | Round; during culture, a small proportion (10%) of spindle-shaped, multi-angular differentiated cells is considered normal. |
Cell Culture Medium | DMEM+10%FBS+1%P/S; |
Culture Environment | CO2 : 5%; Temperature: 37°C |
Medium Change Frequency | Daily |
Passage Ratio | 1:3-1:6 |
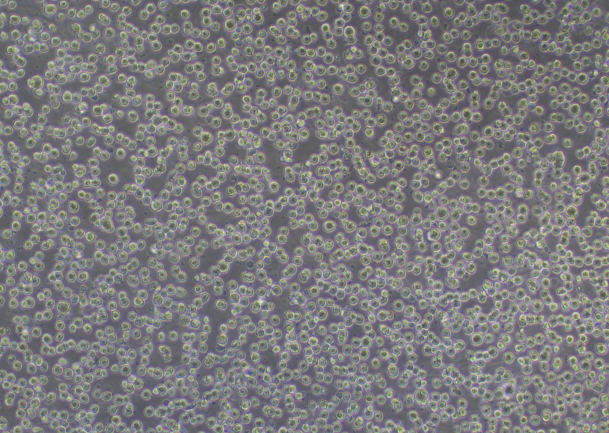
RAW 264.7 Cell Image (Normal Growth)
Cell Thawing Procedure:
1) Preparation: warm up the complete culture medium in 37°C water bath for 30 mins;
2) Inside the ultra-clean bench, pipet 7 ml of complete medium into a 15 ml centrifuge tube;
3) Take out the cryopreserved vial from dry ice, hold the cap with forceps, quickly thaw cells in a 37°C water bath by gently swirling the vial (Note: keep the cap out of the water). In about 1 minute, it would completely thaw;
4) Transfer the thawed cells to the prepared centrifuge tube with complete medium, close the lid, and centrifuge at 1100 rpm for 4 mins at room temp to collect the cells;
5) Inside the ultra-clean bench, carefully remove and discard the supernatant. Resuspend cell pellet with 1ml of complete medium and then transfer to a T25 flask (or 6 cm plate) containing 4 ml of complete medium, label the flask/plate with cell name, date and passage no., incubate the flask in a 37℃, 5%CO2 incubator.
6) Observe the cell status and adhesion the next day.
Key Points for Thawing :
1. The thawing process should not be too long, preferably completed within 15 minutes.
2. If the cell status is poor or there is a lot of differentiation 24 hours after thawing, it is recommended to thaw the cells to a 6-well plate based on the cell count.
3. Do not thaw the cells directly into a T75 cm2 flask or a 10 cm plate.
Cell Passaging Procedure:
1) When the confluence reaches 70-80%, passage can be performed at a ratio of 1:3-1:6;
2) Remove the old culture medium, gently pipet the cells into single cells with fresh complete
culture medium, observe under a microscope if the cells are single cells;
3) Passage the cells according to a certain ratio, and observe the cell status the next
day.
Key Points for Passaging:
1. Do not use trypsin to digest these cells.
2. Differences in serum quality may affect cell growth. It is
recommended to use premium serum for culture. If premium serum is not available, you can increase the serum
concentration for culture.
3. Passage can improve the proportion of
cell differentiation. Due to the rapid growth of RAW 264.7 cells, passage can generally be done in 2-3 days.
Therefore, passages should not exceed three days; exceeding this may make the cells more prone to
differentiation. Differentiated cells adhere firmly to the surface and are difficult to detach, so it is essential
to control the seeding density for each passage.
4. If the
proportion of differentiated cells is significant, when the cells reach 70-80% confluence, use a single-channel
pipette to gently pipet the undifferentiated round cells and
transfer them to a new culture plate and pipet them into single cells (note the seeding density, not too
sparse). Pipet only once to avoid the differentiated cells also collected for further culture, and through this
operation in 2-3 passages as described above, normal cells can be obtained.
Important Tips With An Example:
If there is a high proportion of differentiated cells in a T25
flask, gently pipet the surface of the cells with 2-3 ml of
medium, be careful not to create air bubbles. Collect the undifferentiated cells and transfer them to a suitable
plate. Under a microscope, check if the cells are single cells. If they are, place them in the incubator. In about
3-4 hours, the cells will adhere to 80-90%, and through observation at this time, if most cells are individual and round, the cluster formation and differentiated cells should be redued in the next day.
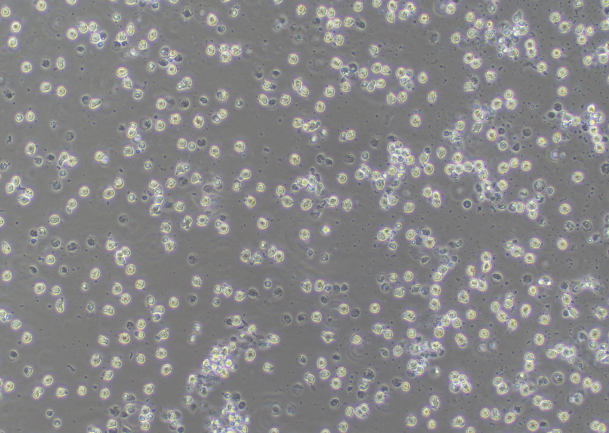
RAW 264.7 Cell Image (Differentiated)
Cell Cryopreservation Procedure:
1) Same as procedures of cell passaging, pipet the cells to a single-cell suspension, and transfer to a centrifuge tube;
2) Mix well by pipetting and perform cell counting;
3) Centrifuge at 1100 rpm for 4 mins at room temp. After centrifugation, remove and discard the supernatant, and resuspend the cells with 4℃ pre-cooled cryopreservation medium, then add cryopreservation medium to adjust to the required density (1x106-1x107 cells/ml);
4) Aliquot the cell suspension to cryovials as 1 ml/tube, place the cryovials in 4℃ pre-cooled Freezing Container, then put the container in an ultra-low temperature freezer;
5) Stay overnight, transfer the cryovials to liquid nitrogen for long-term storage.
After learning from the shared expert insights, you should feel more capable of addressing the differentiation issue with RAW 264.7 cells. If you found this useful, feel free to share it with your colleagues who culture RAW 264.7 cells!
Ubigene's 200+ Wild-Type Cell Lines have been selected through thousands of gene-editing experiments, featuring low passage numbers, high activity, and good status. This can effectively reduce experimental trial and error costs and shorten the experimental period. All human and mouse cell lines in our cell bank can provide STR authentification reports to ensure accurate cell identity. Product information has been included in the ExPASy Cellosaurus database, guaranteeing quality.